Exam 10: Structure and Synthesis of Alcohols
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Given the set of reactants below, complete the acid-base reaction, and indicate whether the equilibrium favors reactants or products.
CH3O- + HCl
(Essay)
4.8/5  (31)
(31)
Which of the following reagents or sequences do not produce an alcohol or diol from an alkene starting material?
(Multiple Choice)
5.0/5  (40)
(40)
Explain how a mixture of phenol and cyclopentanol might be separated using differences in their solubility properties.
(Essay)
4.9/5  (40)
(40)
Ethanol that contains added impurities that make it unfit for drinking is described as ________.
(Short Answer)
4.8/5  (46)
(46)
Provide the structure of the major organic product in the reaction shown below. 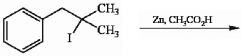
(Essay)
4.8/5  (39)
(39)
Provide the reagents necessary to accomplish the following transformation.
2-methyl-2-octene to 2-methyloctan-3-ol
(Essay)
4.7/5  (32)
(32)
In a 1-butanol molecule, what part of the molecule is described as hydrophilic?
(Short Answer)
4.9/5  (29)
(29)
________ is the major intermolecular attraction responsible for the relatively high boiling points of alcohols.
(Short Answer)
4.8/5  (34)
(34)
Name the major organic product which results when CH3CO2CH2CH3 is treated with 2 equivalents of (CH3)2CHMgBr followed by protonation with dilute acid?
(Short Answer)
4.8/5  (46)
(46)
Distillation of mixtures of ethanol and water cannot increase the ethanol content of the mixture above 95% because this solution boils at a lower temperature than either pure ethanol or pure water. The term which describes this lower boiling mixture is ________.
(Short Answer)
4.8/5  (39)
(39)
Provide the structure of the major organic product in the reaction shown below. 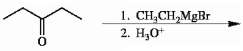
(Essay)
4.8/5  (39)
(39)
Which of the following solvents is best-suited to the preparation of a Grignard reagent: hexane, diethyl ether, or ethanol? Explain.
(Essay)
4.8/5  (24)
(24)
Provide the name of the major organic product that results when 1-pentene is treated with aqueous acid.
(Short Answer)
4.8/5  (37)
(37)
How would one used a Grignard-based synthesis to accomplish the following transformation?
pentanal (CH3CH2CH2CH2CHO) to heptan-3-ol
(Essay)
4.8/5  (39)
(39)
Showing 61 - 80 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)