Multiple Choice
Nitrogen dioxide decomposes according to the reaction 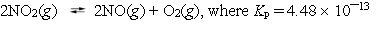 at 25 C. What is the value for Kc?
at 25 C. What is the value for Kc?
A) 1.81 * 10¯16
B) 1.83 * 10¯14
C) 4.48 *10¯13
D) 1.10 *10¯11
E) 1.11 * 10¯9
Correct Answer:

Verified
Correct Answer:
Verified
Q31: A good catalyst for a reaction will
Q53: The reaction of nitric oxide to
Q54: A mixture of 0.500 mole of carbon
Q55: The equilibrium constant, K<sub>p</sub>, for the
Q56: Consider the reversible reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q57: Carbon monoxide and chlorine combine in an
Q59: At high temperatures, carbon reacts with O<sub>2</sub>
Q60: What is the mass-action expression, Q<sub>c</sub>, for
Q61: Write the mass-action expression, Q<sub>c</sub>, for the
Q62: What is the mass-action expression, Q<sub>p</sub>, for