Essay
Calculate the COCl2, CO, and Cl2 concentrations when the following gas-phase reaction reaches equilibrium at 300°C.
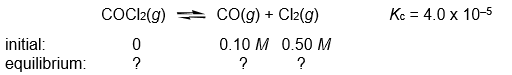
Correct Answer:

Verified
[CO] = 1 x...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: In which of the following will the
Q11: If the equilibrium constant for the following
Q12: Under which set of conditions must the
Q13: Which is the correct equilibrium constant expression
Q14: Hidden Assumptions that make Equilibrium Calculations Easier
Q16: Which of the following factors will
Q17: For the following reaction:<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q18: Based on the information given in the
Q19: Which of the following graphs best represents
Q20: Assume that the equilibrium constant for the