Multiple Choice
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 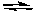 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with 0.10 M H2O(g) , what would be the best strategy for solving for the equilibrium concentrations of H2O, H2 and O2?
A) Since the change in the concentrations are expected to be large going to equilibrium, the equation will have to be solved exactly.
B) Since the changes in the concentrations are expected to be large going to equilibrium, it is best to assume that all of the H2O(g) is converted to products and then calculate the equilibrium concentration.
C) Since the changes in concentrations are expected to be small going to equilibrium, it is best to assume that the changes will be small relative to the initial concentration of H2O(g) .
D) Since the changes in concentrations are expected to be small going to equilibrium, it is best to assume that the changes will be large relative to the initial concentration of H2O(g) .
E) There is no reasonable way to even approximate the answer to this problem.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Calculate the concentrations of Cl<sub>2</sub> and ClF<sub>3</sub>
Q10: In which of the following will the
Q11: If the equilibrium constant for the following
Q12: Under which set of conditions must the
Q13: Which is the correct equilibrium constant expression
Q15: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q16: Which of the following factors will
Q17: For the following reaction:<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q18: Based on the information given in the
Q19: Which of the following graphs best represents