Multiple Choice
For the following reaction:
2 NOCl(g) 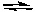 2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO2) = 0.989 M at some moment in time, which of the following would be true?
A) The rate of the forward reaction would be exactly equal to the rate of the reverse reaction.
B) Neither the forward nor the reverse reaction would occur because the reaction would be at equilibrium.
C) The rate of the forward reaction would be faster than the reverse reaction.
D) The rate of the reverse reaction would be faster than the forward reaction.
E) There is no relationship between these concentrations and the rate of either the forward or the reverse reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Under which set of conditions must the
Q13: Which is the correct equilibrium constant expression
Q14: Hidden Assumptions that make Equilibrium Calculations Easier
Q15: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q16: Which of the following factors will
Q18: Based on the information given in the
Q19: Which of the following graphs best represents
Q20: Assume that the equilibrium constant for the
Q21: A 50.0 mL solution of 0.015 M
Q22: Which is the correct reaction for the