Multiple Choice
What would happen to the extent of the decomposition of PCl5 at a given temperature if the pressure on the system were decreased?
PCl5(g) 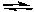 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
A) It would increase.
B) It would decrease.
C) It would remain constant.
D) Not enough information is given to determine this.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Which of the following changes will
Q33: For the reaction<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="For
Q34: The equilibrium constant for the following reaction
Q35: If at any moment in time, we
Q36: Which of the following equations correctly describes
Q38: Hidden Assumptions that make Equilibrium Calculations Easier
Q39: Hidden Assumptions that make Equilibrium Calculations
Q40: Hidden Assumptions that make Equilibrium Calculations Easier
Q41: The addition of I<sub>2</sub>(g) to a fixed
Q42: A solution is prepared in which the