Multiple Choice
Calculate Hrxn° for the following reaction from the data given below. 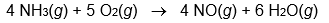
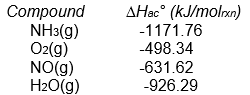
A) -105.5 kJ/molrxn
B) -905.5 kJ/molrxn
C) -1274.2 kJ/molrxn
D) -1996.2 kJ/molrxn
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Which of the following reactions is
Q42: (Note that some of these
Q43: The following questions often assume that a
Q44: (Note that some of these
Q45: Calculate <span class="ql-formula" data-value="\Delta"><span
Q47: Use enthalpies of atom combination to calculate
Q48: (Note that some of these
Q49: (Note that some of these
Q50: Hydrogen peroxide is a good oxidizing
Q51: The change in enthalpy, <span