Essay
Use enthalpies of atom combination to calculate the change in enthalpy for
the reaction:
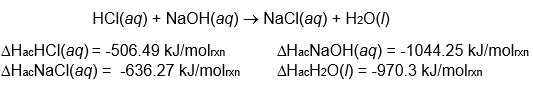
Is the reaction endothermic or exothermic?
Correct Answer:

Verified
-55.8 kJ/m...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
-55.8 kJ/m...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q42: (Note that some of these
Q43: The following questions often assume that a
Q44: (Note that some of these
Q45: Calculate <span class="ql-formula" data-value="\Delta"><span
Q46: Calculate <span class="ql-formula" data-value="\Delta"><span
Q48: (Note that some of these
Q49: (Note that some of these
Q50: Hydrogen peroxide is a good oxidizing
Q51: The change in enthalpy, <span
Q52: (Note that some of these