Multiple Choice
When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below.CO(g) + 3H2(g) 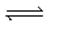 CH4(g) + H2O(g)
CH4(g) + H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve C? 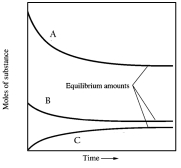
A) hydrogen
B) carbon monoxide
C) either methane or water
D) either hydrogen or carbon monoxide
E) not enough information to decide
Correct Answer:

Verified
Correct Answer:
Verified
Q39: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is
Q40: Consider the following equilibrium:<br>CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q42: Assume that the following chemical reaction
Q43: What is the reaction quotient,Q,for the equilibrium<br><img
Q45: In an experiment,0.42 mol H<sub>2</sub> and 0.42
Q46: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q47: The reaction quotient,Q,for a system is <img
Q48: What is the K<sub>c</sub> expression for the
Q49: For the reaction NO(g)+ ½ O<sub>2</sub>(g)