Multiple Choice
At a certain elevation, the boiling point of water is 98.5 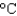 . How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol ·
. How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol · 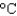 ) , d 1.00 g/mL)
) , d 1.00 g/mL)
A) 8.5 kJ
B) 11.0 kJ
C) 0.93 kJ
D) 1.0 kJ
E) 25.6 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q122: Assuming that the charge of the ions
Q123: Use the following information to determine the
Q124: In terms of the enthalpy of formation,
Q125: A 150 g piece of iron (C<sub>P</sub>
Q126: Using the following data for water, determine
Q128: A 15 g piece of iron (C<sub>P</sub>
Q129: Which statement A-D about a system and
Q130: Use the following information to determine the
Q131: For which reaction below does the enthalpy
Q132: Which of the following fuels has the