Multiple Choice
Using the following data for water, determine the final temperature when 100 g of ice at 10 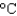 is heated with 350 kJ of energy. Boiling point
is heated with 350 kJ of energy. Boiling point
373 K
Melting point
273 K
Enthalpy of vaporization
2,260 J/g
Enthalpy of fusion
334 J/g
Specific heat capacity (solid)
2) 11 J/(g K)
Specific heat capacity (liquid)
4) 18 J/(g K)
Specific heat capacity (gas)
2) 08 J/(g K)
A) 309 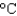
B) 100 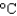
C) 382 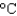
D) 225 
E) 325 
Correct Answer:

Verified
Correct Answer:
Verified
Q121: Indicate which of the following is not
Q122: Assuming that the charge of the ions
Q123: Use the following information to determine the
Q124: In terms of the enthalpy of formation,
Q125: A 150 g piece of iron (C<sub>P</sub>
Q127: At a certain elevation, the boiling point
Q128: A 15 g piece of iron (C<sub>P</sub>
Q129: Which statement A-D about a system and
Q130: Use the following information to determine the
Q131: For which reaction below does the enthalpy