Multiple Choice
In terms of the enthalpy of formation, which of the following compounds is the most unstable compared to its elements under standard conditions?
A) KCl(s) , 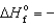 435.9 kJ/mol
435.9 kJ/mol
B) PH3(g) , 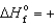 5.4 kJ/mol
5.4 kJ/mol
C) Fe3O4(s) , 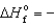 1,117.1 kJ/mol
1,117.1 kJ/mol
D) NiO(s) , 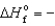 239.7 kJ/mol
239.7 kJ/mol
E) ICl(g) , 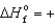 17.8 kJ/mol
17.8 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q119: When a 13.0 g sample of NaOH(s)
Q120: The cooling system in an automobile holds
Q121: Indicate which of the following is not
Q122: Assuming that the charge of the ions
Q123: Use the following information to determine the
Q125: A 150 g piece of iron (C<sub>P</sub>
Q126: Using the following data for water, determine
Q127: At a certain elevation, the boiling point
Q128: A 15 g piece of iron (C<sub>P</sub>
Q129: Which statement A-D about a system and