Multiple Choice
A 125 mL sample of orange juice was titrated using a redox reaction to the equivalence point with the addition of 7.6 mL of a 0.0025M iodine (I2) solution. What is the concentration of vitamin C (C6H8O6) in this sample? C6H8O6(aq) 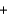 I2(aq)
I2(aq)  C6H6O6(aq)
C6H6O6(aq) 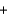 2I-(aq)
2I-(aq) 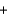 2H+(aq)
2H+(aq)
A) 30 mM
B) 15 mM
C) 19 mM
D) 0.30 mM
E) 0.15 mM
Correct Answer:

Verified
Correct Answer:
Verified
Q55: Which of the following compounds is a
Q56: What volume of 0.25 M hydrochloric acid
Q57: Arsenic, which is toxic, can be removed
Q58: Identify the acid in the following acid-base
Q59: In a spontaneous oxidation reduction reaction between
Q61: The thermite reaction is used in welding
Q62: Dinitrogen monoxide (N<sub>2</sub>O) is produced from nitrate
Q63: Which of the following statements is not
Q64: Which one of the following is the
Q65: In a titration, the solution of known