Multiple Choice
The thermite reaction is used in welding applications and chemistry demonstrations because it releases a tremendous amount of energy. Which of the following statements is not correct regarding this reaction? 8Al 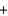 3Fe3O4
3Fe3O4  9Fe
9Fe 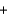 4Al2O3
4Al2O3
A) Aluminum is oxidized.
B) Iron is reduced.
C) The oxidation number of Al changes from 0 to +3.
D) Aluminum is the reducing agent.
E) Three electrons are transferred from each aluminum atom to each iron atom.
Correct Answer:

Verified
Correct Answer:
Verified
Q56: What volume of 0.25 M hydrochloric acid
Q57: Arsenic, which is toxic, can be removed
Q58: Identify the acid in the following acid-base
Q59: In a spontaneous oxidation reduction reaction between
Q60: A 125 mL sample of orange juice
Q62: Dinitrogen monoxide (N<sub>2</sub>O) is produced from nitrate
Q63: Which of the following statements is not
Q64: Which one of the following is the
Q65: In a titration, the solution of known
Q66: Some microorganisms living under anaerobic conditions extract