Multiple Choice
Dinitrogen monoxide (N2O) is produced from nitrate ions in aqueous solution by anaerobic bacteria. Which one of the following statements about this reaction is not correct? 2NO3-(aq) 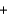 H2O(l )
H2O(l )  N2O(g)
N2O(g) 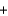 2O2(g)
2O2(g) 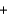 2OH-(aq)
2OH-(aq)
A) Nitrogen is reduced.
B) Oxygen is oxidized.
C) Water can be written as a reactant in the oxidation half reaction.
D) Nitrate is the oxidizing agent.
E) As written above, four electrons are transferred from oxygen to nitrogen in this reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q57: Arsenic, which is toxic, can be removed
Q58: Identify the acid in the following acid-base
Q59: In a spontaneous oxidation reduction reaction between
Q60: A 125 mL sample of orange juice
Q61: The thermite reaction is used in welding
Q63: Which of the following statements is not
Q64: Which one of the following is the
Q65: In a titration, the solution of known
Q66: Some microorganisms living under anaerobic conditions extract
Q67: Which of the following compounds are soluble