Multiple Choice
Which of the following statements is not correct regarding the following redox reaction? MnO2 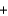 4HCl
4HCl  MnCl2
MnCl2 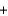 Cl2
Cl2
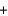 2H2O
2H2O
A) Manganese(IV) oxide is the oxidizing agent.
B) Chloride is oxidized.
C) The oxidation number of Mn changes from +4 to +2.
D) The oxidation number of Cl changes from -1 to 0.
E) One electron is transferred from manganese to each chloride.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Identify the acid in the following acid-base
Q59: In a spontaneous oxidation reduction reaction between
Q60: A 125 mL sample of orange juice
Q61: The thermite reaction is used in welding
Q62: Dinitrogen monoxide (N<sub>2</sub>O) is produced from nitrate
Q64: Which one of the following is the
Q65: In a titration, the solution of known
Q66: Some microorganisms living under anaerobic conditions extract
Q67: Which of the following compounds are soluble
Q68: Human blood contains about 0.18 g potassium