Exam 22: Carbonyl Alpha-Substitution Reactions
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
Exhibit 22-1
Refer to the compounds below to answer the following question(s): 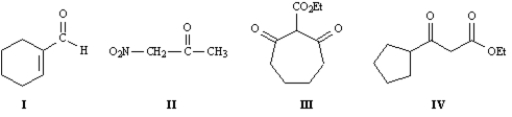 -Refer to Exhibit 22-1.Indicate all the acidic hydrogens in Compounds I through IV.
-Refer to Exhibit 22-1.Indicate all the acidic hydrogens in Compounds I through IV.
(Essay)
4.8/5  (24)
(24)
Draw the general mechanism of the α-substitution reaction between acetone and the electrophilic methyl group.
(Essay)
4.8/5  (29)
(29)
Draw the mechanism of enol formation from acetone in a reaction catalyzed by hydrochloric acid.
(Essay)
4.8/5  (32)
(32)
Exhibit 22-4
Consider the reaction sequence below to answer the following question(s): 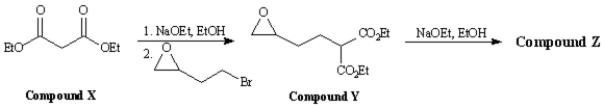 -Refer to Exhibit 22-4.Compound X,diethyl propanedioate,is more commonly known as _____.
-Refer to Exhibit 22-4.Compound X,diethyl propanedioate,is more commonly known as _____.
(Multiple Choice)
4.9/5  (31)
(31)
Explain why the following reaction does not occur to any significant extent respect when the reactant is treated with bromine in the presence of acetic acid.Atoms other than carbon and hydrogen are labeled.
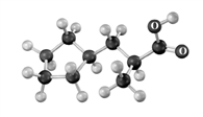
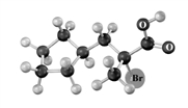 Reactant Product
Reactant Product
(Essay)
4.8/5  (37)
(37)
The following substance is produced using acetoacetic ester synthesis.Atoms other than carbon and hydrogen are labeled. 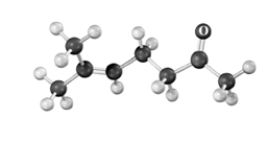 Which of the following regions of an IR spectrum could be used to monitor the progress of the reaction?
Which of the following regions of an IR spectrum could be used to monitor the progress of the reaction?
(Multiple Choice)
4.8/5  (40)
(40)
Exhibit 22-3
Consider the reaction below to answer the following question(s). 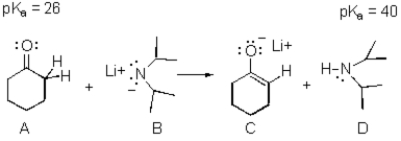 -Refer to Exhibit 22-3.The weakest acid in the reaction is:
-Refer to Exhibit 22-3.The weakest acid in the reaction is:
(Short Answer)
4.9/5  (42)
(42)
Exhibit 22-6
Consider the reaction below to answer the following question(s): 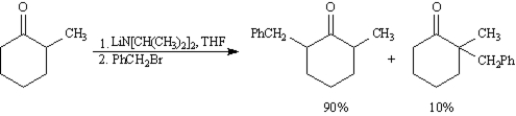 -Refer to Exhibit 22-6.Explain the product ratio in this reaction.
-Refer to Exhibit 22-6.Explain the product ratio in this reaction.
(Essay)
4.8/5  (47)
(47)
Using the acetoacetic ester synthesis,to produce 5-methyl-2-heptanone,the alkyl halide that should be used is:
(Multiple Choice)
4.8/5  (33)
(33)
Exhibit 22-3
Consider the reaction below to answer the following question(s).  -Refer to Exhibit 22-3.The enolate ion in the reaction is:
-Refer to Exhibit 22-3.The enolate ion in the reaction is:
(Short Answer)
4.9/5  (40)
(40)
Explain how to use an alkylation reaction to produce: 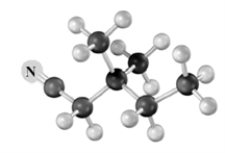 from
from 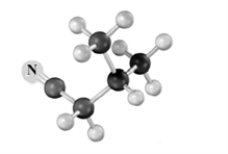 Atoms other than carbon and hydrogen are labeled.
Atoms other than carbon and hydrogen are labeled.
(Essay)
4.9/5  (44)
(44)
Draw the mechanism of the acid-catalyzed chlorination of acetophenone in the presence of a base.
(Essay)
4.8/5  (38)
(38)
Showing 21 - 33 of 33
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)