Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
The amount of energy in electromagnetic radiation is related to the frequency and wavelength of the radiation.High energy radiation like gamma rays is of:
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following provides information about the functional groups present in a sample?
(Multiple Choice)
4.9/5  (37)
(37)
What m/z ratio would expect to find in the mass spectrum of the following compound due to dehydration? 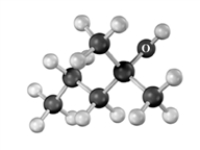
(Multiple Choice)
4.9/5  (40)
(40)
Both mass spectrometry and infrared spectroscopy involve the interaction of molecules with electromagnetic energy?
(True/False)
4.9/5  (37)
(37)
Exhibit 12-6
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
-_____ C−C,C−O,C−N,and C−X single-bond vibrations.
(Multiple Choice)
4.8/5  (36)
(36)
Consider the following spectrum. 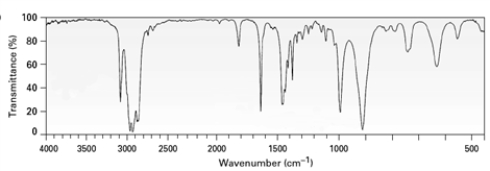 The compound used to generate this spectrum might have which of the following molecular formulas?
The compound used to generate this spectrum might have which of the following molecular formulas?
(Multiple Choice)
4.8/5  (38)
(38)
When the molecular ion of 2,2-dimethylpentane fragments,the positive charge is most likely to stay on:
(Multiple Choice)
4.8/5  (38)
(38)
Exhibit 12-1
Select the most reasonable formula for the compounds with the following mass spectral data.
-Refer to Exhibit 12-1.M+ m/z = 136 and M+ at m/z = 138 of approximately equal intensity
(Multiple Choice)
4.9/5  (28)
(28)
Exhibit 12-5
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). 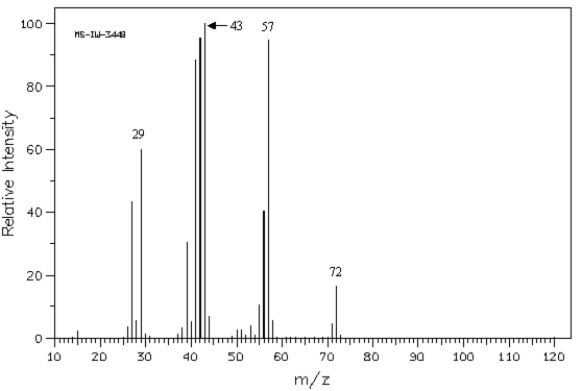 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
-Refer to Exhibit 12-5.Propose structures for fragment ions at m/z = 57,43,and 29.
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
-Refer to Exhibit 12-5.Propose structures for fragment ions at m/z = 57,43,and 29.
(Essay)
4.8/5  (33)
(33)
The sugar glucose contains C,H,and O and has a mass of 180.0634 amu as determined by high-resolution mass spectrometry.Glucose contains an equal number of carbon and oxygen atoms.What is the molecular formula of glucose? (1H = 1.00783 amu,12C = 12.00000 amu,16O = 15.99491 amu)
(Essay)
4.8/5  (41)
(41)
Cyclohexene and 2-hexyne both have the molecular formula C6H10.How would you use infrared spectroscopy to distinguish between the two compounds?
(Essay)
4.7/5  (31)
(31)
Exhibit 12-2
Use the data below to answer the following question(s).
Loratidine is the active ingredient in the antihistamine Claritin .Mass spectral analysis of loratidine shows M+ at m/z = 382 and M+ at m/z = 384 in an approximate ratio of 3:1 in intensity.
-Refer to Exhibit 12-2.Loratidine is known to contain nitrogen.What is the minimum number of nitrogens in loratidine?
(Essay)
4.9/5  (41)
(41)
Exhibit 12-4
The following question(s) refer to the mass spectrum shown below. 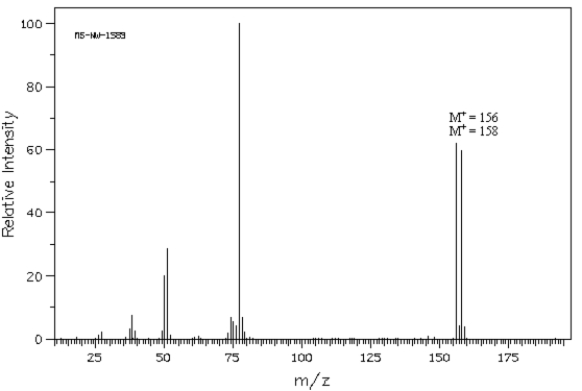 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
-Refer to Exhibit 12-4.Calculate a possible molecular formula for this compound.
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
-Refer to Exhibit 12-4.Calculate a possible molecular formula for this compound.
(Essay)
4.8/5  (37)
(37)
Which of the following statements best describes the base peak in a mass spectrum?
(Multiple Choice)
4.8/5  (43)
(43)
Consider the following mass spectrum 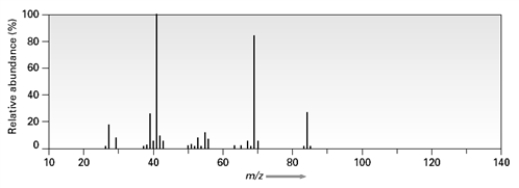 Which of the following compounds might have been used to produce this spectrum?
Which of the following compounds might have been used to produce this spectrum?
(Multiple Choice)
4.9/5  (41)
(41)
Examining the infrared spectrum of a compound allows us to:
(Multiple Choice)
4.9/5  (42)
(42)
Exhibit 12-6
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
-_____ N−H,C−H,and O−H stretching and bending motions.
(Multiple Choice)
4.9/5  (32)
(32)
Could you distinguish 2,2-dimethylbutane from 2-methylpentane using mass spectrometry?
(Essay)
4.8/5  (32)
(32)
The peak in the IR absorption spectra for the alkyne group in acetylene would be observed in the range of:
(Multiple Choice)
5.0/5  (48)
(48)
Below is the mass spectrum of an unknown hydrocarbon.In addition,this hydrocarbon shows characteristic absorption at 2100 cm−1 in its IR spectrum.Give the structure of this unknown. 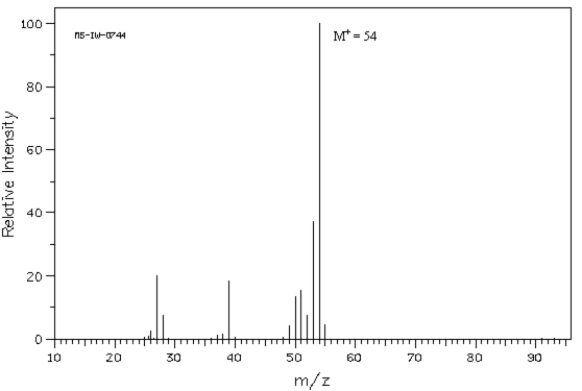 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
(Essay)
4.9/5  (47)
(47)
Showing 21 - 40 of 42
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)