Exam 1: Structure and Bonding
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
Convert the following molecular model into a condensed structure and a skeletal structure. 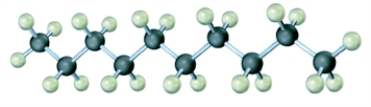
(Essay)
4.9/5  (46)
(46)
Exhibit 1-4
Propose possible structures for a molecule that meets each of the following descriptions.
-Refer to Exhibit 1-4.Contains two sp3 hybridized carbons and two sp hybridized carbons.
(Essay)
4.9/5  (45)
(45)
Exhibit 1-4
Propose possible structures for a molecule that meets each of the following descriptions.
-Refer to Exhibit 1-4.Contains one sp3 hybridized carbon and two sp2 hybridized carbons.
(Essay)
4.9/5  (40)
(40)
Exhibit 1-2
Consider the structure of urea,shown below,to answer the following question(s). 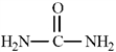 -Refer to Exhibit 1-2.Fill in any non-bonding valence electrons that are missing from the line-bond structure.
-Refer to Exhibit 1-2.Fill in any non-bonding valence electrons that are missing from the line-bond structure.
(Essay)
4.8/5  (32)
(32)
Exhibit 1-2
Consider the structure of urea,shown below,to answer the following question(s). 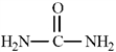 -Refer to Exhibit 1-2.The predicted NH2−C=O bond angle in urea is:
-Refer to Exhibit 1-2.The predicted NH2−C=O bond angle in urea is:
(Multiple Choice)
4.9/5  (40)
(40)
Indicate the hybridization on each of the carbon atoms indicated with a number in the molecular model shown. 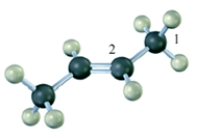
(Essay)
4.8/5  (29)
(29)
Exhibit 1-3
Determine the hybridization for the indicated atoms in each structure below. 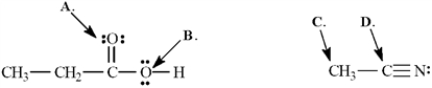 -Refer to Exhibit 1-3.The hybridization of this oxygen atom (A) is ______.
-Refer to Exhibit 1-3.The hybridization of this oxygen atom (A) is ______.
(Short Answer)
5.0/5  (38)
(38)
Exhibit 1-3
Determine the hybridization for the indicated atoms in each structure below. 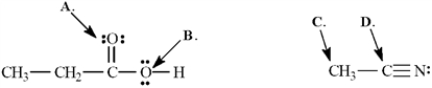 -Refer to Exhibit 1-3.The hybridization of this carbon atom (D) is ______.
-Refer to Exhibit 1-3.The hybridization of this carbon atom (D) is ______.
(Short Answer)
4.9/5  (39)
(39)
Fill in any nonbonding valence electrons that are missing from the following structural representation of the amino acid cysteine. 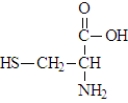
(Essay)
4.8/5  (36)
(36)
Showing 21 - 29 of 29
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)