Exam 41: Atomic Physics
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions49 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics II: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics38 Questions
Exam 42: Nuclear Physics64 Questions
Select questions type
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.Which of the following angles could NOT be the angle between the orbital angular momentum vector of the electron and an arbitrary z-direction?
(Multiple Choice)
4.9/5  (37)
(37)
In the ground state,the quantum numbers (n,l,ml,ms)for hydrogen are,respectively
(Multiple Choice)
4.8/5  (33)
(33)
For each value of the principal quantum number n,what are the possible values of the electron spin quantum number ms? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (39)
(39)
The only VALID electron state and shell designation among the following is
(Multiple Choice)
4.8/5  (36)
(36)
An electron in a hydrogen atom has orbital quantum number l = 7.How many possible values of the magnetic quantum number ml could it have?
(Multiple Choice)
4.9/5  (35)
(35)
What is the correct electronic configuration for ground state carbon,which has 6 electrons?
(Multiple Choice)
4.9/5  (40)
(40)
If the orbital quantum number is l = 4,which one of the following is a possible value for the principal quantum number n?
(Multiple Choice)
4.8/5  (36)
(36)
An electron in a hydrogen atom has principal quantum number n = 4.How many possible values of the orbital quantum number l could it have?
(Multiple Choice)
4.9/5  (41)
(41)
In a ruby laser,an electron jumps from a higher energy level to a lower one.If the energy difference between the two levels is 1.8 eV,what is the wavelength of the emitted photon? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (42)
(42)
Consider the four quantum numbers of an electron in an atom,n,l,ml,and ms.The energy of an electron in an isolated atom depends on
(Multiple Choice)
4.9/5  (43)
(43)
How many photons per second emerge from a laser of power 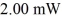 with wavelength
with wavelength 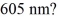 (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.7/5  (33)
(33)
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.Which one of the following numbers could be the principal quantum number n of the electron?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following are characteristics of laser light? (There may be more than one correct choice.)
(Multiple Choice)
4.7/5  (41)
(41)
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.A magnetic field is applied,defining the z-axis along the field.Which of the following sets of angles are possible angles between the magnetic field and the orbital angular momentum?
(Multiple Choice)
4.8/5  (34)
(34)
The only INVALID electron state and shell designation among the following is
(Multiple Choice)
4.8/5  (36)
(36)
What is the correct electronic configuration for the ground state sodium atom,which has 11 electrons?
(Multiple Choice)
4.8/5  (37)
(37)
What is the energy of an incident photon that is just enough to excite a hydrogen atom from its ground state to its n = 4 state?
(Multiple Choice)
5.0/5  (28)
(28)
The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r)= 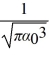 e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 21 - 38 of 38
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)