Exam 13: Principles of Bioenergetics
Exam 1: The Foundations of Biochemistry56 Questions
Exam 2: Water41 Questions
Exam 3: Amino Acids, peptides, and Proteins72 Questions
Exam 4: The Three-Dimensional Structure of Proteins61 Questions
Exam 5: Protein Function45 Questions
Exam 6: Enzymes67 Questions
Exam 7: Carbohydrates and Glycobiology53 Questions
Exam 8: Nucleotides and Nucleic Acids60 Questions
Exam 9: Recombinant Dna Technology46 Questions
Exam 10: Lipids46 Questions
Exam 11: Biological Membranes and Transport60 Questions
Exam 12: Biosignaling70 Questions
Exam 13: Principles of Bioenergetics53 Questions
Exam 14: Glycolysis, gluconeogenesis, and the Pentose Phosphate Pathway79 Questions
Exam 15: Principles of Metabolic Regulation48 Questions
Exam 16: The Citric Acid Cycle58 Questions
Exam 17: Fatty Acid Catabolism50 Questions
Exam 18: Amino Acid Oxidation and the Production of Urea48 Questions
Exam 19: Oxidative Phosphorylation and Photophosphorylation78 Questions
Exam 20: Carbohydrate Biosynthesis in Plants and Bacteria47 Questions
Exam 21: Lipid Biosynthesis58 Questions
Exam 22: Biosynthesis of Amino Acids, nucleotides, and Related Molecules64 Questions
Exam 23: Integration and Hormonal Regulation of Mammalian Metabolism55 Questions
Exam 24: Genes and Chromosomes46 Questions
Exam 25: Dna Metabolism60 Questions
Exam 26: Rna Metabolism58 Questions
Exam 27: Protein Metabolism59 Questions
Exam 28: Regulation of Gene Expression57 Questions
Select questions type
During transfer of two electrons through the mitochondrial respiratory chain,the overall reaction is:
NADH + 1/2 O2 + H+ NAD+ + H2O
For this reaction,the difference in reduction potentials for the two half-reactions ( E'°)is +1.14 V.Show how you would calculate the standard free-energy change, G'°,for the reaction.(The Faraday constant,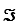 ,is 96.48 kJ/V·mol. )
,is 96.48 kJ/V·mol. )
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
G'° = -n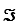 E'°
E'°
For reactions involving NADH,two electrons are transferred (n = 2).So G'° = (-2)(96.48 kJ/V·mol)(1.14 V)= -220 kJ/mol.
Classify each of the *ed atoms as an electrophile or a nucleophile: 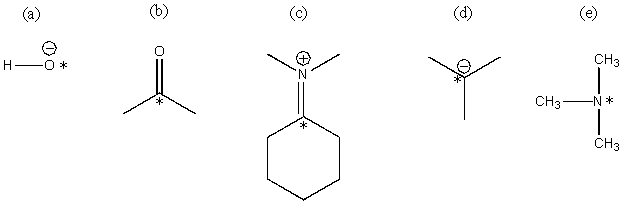
Free
(Short Answer)
4.9/5  (43)
(43)
Correct Answer:
(a)Nucleophile
(b)Electrophile
(c)Electrophile
(d)Nucleophile
(e)Nucleophile
The immediate precursors of DNA and RNA synthesis in the cell all contain:
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
B
Explain in quantitative terms the circumstances under which the following reaction can proceed.
Citrate isocitrate G'° = +13.3 kJ/mol
(Essay)
4.8/5  (30)
(30)
Which one of the following compounds does not have a large negative free energy of hydrolysis?
(Multiple Choice)
4.9/5  (32)
(32)
When a mixture of 3-phosphoglycerate and 2-phosphoglycerate is incubated at 25 °C with phosphoglycerate mutase until equilibrium is reached,the final mixture contains six times as much 2-phosphoglycerate as 3-phosphoglycerate.Which one of the following statements is most nearly correct,when applied to the reaction as written? (R = 8.315 J/mol·K;T = 298 K)
3-Phosphoglycerate 2-phosphoglycerate
(Multiple Choice)
4.8/5  (37)
(37)
The first law of thermodynamics states that the amount of energy in the universe is constant,but that the various forms of energy can be interconverted.Describe four different types of such energy transduction that occur in living organisms and provide one example for each.
(Essay)
4.9/5  (36)
(36)
Consider the reaction: A + B C + D.If the equilibrium constant for this reaction is a large number (say,10,000),what do we know about the standard free-energy change ( G'°)for the reaction? Describe the relationship between Keq' and G'°.
(Essay)
4.7/5  (26)
(26)
The free energy of hydrolysis of phosphoenolpyruvate is -61.9 kJ/mol.Rationalize this large,negative value for G'° in chemical terms.
(Essay)
4.9/5  (37)
(37)
The expression G = G'° + RT ln Keq' for the actual free-energy change for the reaction A + B C + D is incorrect.Why is it wrong,and what is the correct expression for the real free-energy change of this reaction?
(Essay)
4.7/5  (34)
(34)
E'° of the NAD+/NADH half reaction is -0.32 V.The E'° of the oxaloacetate/malate half reaction is
-0)175 V.When the concentrations of NAD+,NADH,oxaloacetate,and malate are all 10-5 M,the "spontaneous" reaction is:
(Multiple Choice)
4.8/5  (28)
(28)
During glycolysis,glucose 1-phosphate is converted to fructose 6-phosphate in two successive reactions:
Glucose 1-phosphate glucose 6-phosphate G'° = -7.1 kJ/mol
Glucose 6-phosphate fructose 6-phosphate G'° = +1.7 kJ/mol
G'° for the overall reaction is:
(Multiple Choice)
4.7/5  (31)
(31)
Glycerol 3-phosphate dehydrogenase catalyzes the following reversible reaction:
Glycerol 3-phosphate + NAD+ NADH + H+ + dihydroxyacetone phosphate
Given the standard reduction potentials below,calculate G'° for the glycerol 3-phosphate dehydrogenase reaction,proceeding from left to right as shown.Show your work.(The Faraday constant,F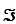 ,is 96.48 kJ/V·mol. )
,is 96.48 kJ/V·mol. )
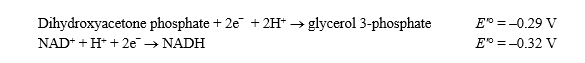
(Essay)
4.9/5  (27)
(27)
For each pair of ions or compounds below,indicate which is the more highly reduced species.
(a)Co2+/Co+
(b)Glucose/CO2
(c)Fe3+/Fe2+
(d)Acetate/CO2
(e)Ethanol/acetic acid
(f)Acetic acid/acetaldehyde
(Short Answer)
4.8/5  (36)
(36)
The standard reduction potentials (E'°)for the following half reactions are given.
Fumarate + 2H+ + 2e- succinate E'° = +0.031 V
FAD + 2H+ + 2e- FADH2 E'° = -0.219 V
If you mixed succinate,fumarate,FAD,and FADH2 together,all at l M concentrations and in the presence of succinate dehydrogenase,which of the following would happen initially?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is true about oxidation-reduction reactions?
(Multiple Choice)
4.8/5  (39)
(39)
In glycolysis,the enzyme pyruvate kinase catalyzes this reaction:
Phosphoenolpyruvate + ADP pyruvate + ATP
Given the information below,show how you would calculate the equilibrium constant for this reaction.(R = 8.315 J/mol·K;T = 298 K)
Reaction 1)ATP ADP + Pi G'° = -30.5 kJ/mol
Reaction 2)phosphoenolpyruvate pyruvate + Pi G'° = -61.9 kJ/mol
(Essay)
4.9/5  (32)
(32)
If E'° for an oxidation-reduction reaction is positive,will G'° be positive or negative? What is the equation that relates G'° and E'°?
(Short Answer)
4.8/5  (19)
(19)
Showing 1 - 20 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)