Exam 13: Principles of Bioenergetics
Exam 1: The Foundations of Biochemistry56 Questions
Exam 2: Water41 Questions
Exam 3: Amino Acids, peptides, and Proteins72 Questions
Exam 4: The Three-Dimensional Structure of Proteins61 Questions
Exam 5: Protein Function45 Questions
Exam 6: Enzymes67 Questions
Exam 7: Carbohydrates and Glycobiology53 Questions
Exam 8: Nucleotides and Nucleic Acids60 Questions
Exam 9: Recombinant Dna Technology46 Questions
Exam 10: Lipids46 Questions
Exam 11: Biological Membranes and Transport60 Questions
Exam 12: Biosignaling70 Questions
Exam 13: Principles of Bioenergetics53 Questions
Exam 14: Glycolysis, gluconeogenesis, and the Pentose Phosphate Pathway79 Questions
Exam 15: Principles of Metabolic Regulation48 Questions
Exam 16: The Citric Acid Cycle58 Questions
Exam 17: Fatty Acid Catabolism50 Questions
Exam 18: Amino Acid Oxidation and the Production of Urea48 Questions
Exam 19: Oxidative Phosphorylation and Photophosphorylation78 Questions
Exam 20: Carbohydrate Biosynthesis in Plants and Bacteria47 Questions
Exam 21: Lipid Biosynthesis58 Questions
Exam 22: Biosynthesis of Amino Acids, nucleotides, and Related Molecules64 Questions
Exam 23: Integration and Hormonal Regulation of Mammalian Metabolism55 Questions
Exam 24: Genes and Chromosomes46 Questions
Exam 25: Dna Metabolism60 Questions
Exam 26: Rna Metabolism58 Questions
Exam 27: Protein Metabolism59 Questions
Exam 28: Regulation of Gene Expression57 Questions
Select questions type
What is an oxidation? What is a reduction? Can an oxidation occur without a simultaneous reduction? Why or why not?
(Essay)
4.7/5  (28)
(28)
Bioenergetics and thermodynamics
What is the difference between G and G'° of a chemical reaction? Describe,quantitatively,the relationship between them.
(Essay)
4.8/5  (35)
(35)
The hydrolysis of phosphoenolpyruvate proceeds with a G'° of about -62 kJ/mol.The greatest contributing factors to this reaction are the destabilization of the reactants by electostatic repulsion and stabilization of the product pyruvate by:
(Multiple Choice)
4.9/5  (36)
(36)
Why is the actual free energy ( G)of hydrolysis of ATP in the cell different from the standard free energy ( G'°)?
(Essay)
4.9/5  (36)
(36)
If a 0.1 M solution of glucose 1-phosphate is incubated with a catalytic amount of phospho-glucomutase,the glucose 1-phosphate is transformed to glucose 6-phosphate until equilibrium is reached.At equilibrium,the concentration of glucose 1-phosphate is 4.5 x 10-3 M and that of glucose 6-phosphate is 8.6 *10-2 M.Set up the expressions for the calculation of Keq' and G'° for this reaction (in the direction of glucose 6-phosphate formation).(R = 8.315 J/mol·K;T = 298 K)
(Essay)
4.9/5  (35)
(35)
Which of the following is not true for the nicotinamide cofactors?
(Multiple Choice)
4.8/5  (39)
(39)
For the reaction A B, G'° = -60 kJ/mol.The reaction is started with 10 mmol of A;no B is initially present.After 24 hours,analysis reveals the presence of 2 mmol of B,8 mmol of A.Which is the most likely explanation?
(Multiple Choice)
4.9/5  (33)
(33)
The G'° values for the two reactions shown below are given.
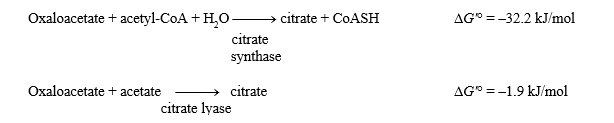 What is the G'° for the hydrolysis of acetyl-CoA?
Acetyl-CoA + H2O acetate + CoASH + H+
What is the G'° for the hydrolysis of acetyl-CoA?
Acetyl-CoA + H2O acetate + CoASH + H+
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following compounds has the largest negative value for the standard free-energy change ( G'°)upon hydrolysis?
(Multiple Choice)
4.8/5  (45)
(45)
Explain why each of the following statements is false.
(a)In a reaction under standard conditions,only the reactants are fixed at 1 M.
(b)When G'° is positive,Keq' > 1.
(c) G and G'° mean the same thing.
(d)When G'° = 1.0 kJ/mol,Keq' = 1.
(Essay)
4.8/5  (38)
(38)
In glycolysis,fructose 1,6-bisphosphate is converted to two products with a standard free-energy change ( G'°)of 23.8 kJ/mol.Under what conditions encountered in a normal cell will the free-energy change ( G)be negative,enabling the reaction to proceed spontaneously to the right?
(Multiple Choice)
4.8/5  (34)
(34)
The standard free energy change ( G'°)for ATP hydrolysis is -30.5 kJ/mol.ATP,ADP,and Pi are mixed together at initial concentrations of 1 M of each,then left alone until the reaction ADP + Pi ATP has come to equilibrium.For each species (i.e. ,ATP,ADP,and Pi),indicate whether the concentration will be equal to 1 M,less than 1 M,or greater than 1 M.
(Essay)
4.9/5  (46)
(46)
For the following reaction, G'° = +29.7 kJ/mol.
L-Malate + NAD+ oxaloacetate + NADH + H+
The reaction as written:
(Multiple Choice)
4.9/5  (39)
(39)
Alcohol dehydrogenase catalyzes the following reversible reaction:
Acetaldehyde + NADH + H+ Ethanol + NAD+
Use the following information to answer the questions below:
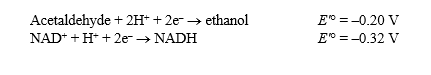 The Faraday constant,
The Faraday constant,  ,is 96.48 kJ/V·mol.
(a)Calculate G'° for the reaction as written.Show your work.
(b)Given your answer to (a),what is the G'° for the reaction occurring in the reverse direction?
(c)Which reaction (forward or reverse)will tend to occur spontaneously under standard conditions?
(d)In the cell,the reaction actually proceeds in the direction that has a positive G'°.Explain how this could be possible.
,is 96.48 kJ/V·mol.
(a)Calculate G'° for the reaction as written.Show your work.
(b)Given your answer to (a),what is the G'° for the reaction occurring in the reverse direction?
(c)Which reaction (forward or reverse)will tend to occur spontaneously under standard conditions?
(d)In the cell,the reaction actually proceeds in the direction that has a positive G'°.Explain how this could be possible.
(Essay)
4.9/5  (33)
(33)
Hydrolysis of 1 M glucose 6-phosphate catalyzed by glucose 6-phosphatase is 99% complete at equilibrium (i.e. ,only 1% of the substrate remains).Which of the following statements is most nearly correct? (R = 8.315 J/mol·K;T = 298 K)
(Multiple Choice)
4.9/5  (33)
(33)
In general,when ATP hydrolysis is coupled to an energy-requiring reaction,the actual reaction often consists of the transfer of a phosphate group from ATP to another substrate,rather than an actual hydrolysis of the ATP.Explain.
(Essay)
4.8/5  (36)
(36)
Lactate dehydrogenase catalyzes the reversible reaction:
Pyruvate + NADH + H+ Lactate + NAD+
Given the following facts
(a)tell in which direction the reaction will tend to go if NAD+,NADH,pyruvate,and lactate were mixed,all at 1 M concentrations,in the presence of lactate dehydrogenase at pH 7; (b)calculate G'° for this reaction.Show your work.
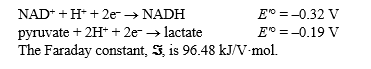
(Essay)
4.9/5  (37)
(37)
Showing 21 - 40 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)