Exam 2: Atoms, molecules, and Ions
Exam 1: Matter and Measurements44 Questions
Exam 2: Atoms, molecules, and Ions59 Questions
Exam 3: Mass Relations in Chemistry;stoichiometry56 Questions
Exam 4: Reactions in Aqueous Solution47 Questions
Exam 5: Gases52 Questions
Exam 6: Electronic Structure and the Periodic Table48 Questions
Exam 7: Covalent Bonding59 Questions
Exam 8: Thermochemistry48 Questions
Exam 9: Liquids and Solids52 Questions
Exam 10: Solutions52 Questions
Exam 11: Rate of Reaction44 Questions
Exam 12: Gaseous Chemical Equilibrium49 Questions
Exam 13: Acids and Bases57 Questions
Exam 14: Equilibria in Acid-Base Solutions49 Questions
Exam 15: Complex Ion and Precipitation Equilibria59 Questions
Exam 16: Spontaneity of Reaction49 Questions
Exam 17: Electrochemistry52 Questions
Exam 18: Nuclear Reactions51 Questions
Exam 19: Complex Ions51 Questions
Exam 20: Chemistry of the Metals51 Questions
Exam 21: Chemistry of the Nonmetals52 Questions
Exam 22: Organic Chemistry51 Questions
Exam 23: Organic Polymers, natural and Synthetic43 Questions
Select questions type
Of the naturally occurring elements in group 14,how many are nonmetals,metalloids,and metals?
(Multiple Choice)
4.9/5  (39)
(39)
Using the laws of constant composition and the conservation of mass,complete the molecular picture of hydrogen molecules (circles)reacting with chlorine molecules (squares)to give hydrogen chloride (HCl). 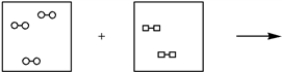
(Multiple Choice)
4.7/5  (38)
(38)
Sodium sulfate has the chemical formula Na2SO4.Based on this information,the formula for chromium(III)sulfate is ____.
(Multiple Choice)
4.8/5  (33)
(33)
The average molar mass of lithium is 6.941.A sample of lithium consists of two isotopes with masses of 6.01512 amu and 7.01600 amu.Determine the percent abundance of each isotope.
(Multiple Choice)
4.9/5  (44)
(44)
Which element is most likely to form an ion with a −2 charge?
(Multiple Choice)
4.8/5  (32)
(32)
What is the symbol for an element which contains 57 neutrons and has a mass number of 101?
(Multiple Choice)
4.7/5  (33)
(33)
Two isotopes of chlorine are found in nature,Cl-35 and Cl-37.The average mass of chlorine is 35.45 amu.The more abundant isotope of Cl has
(Multiple Choice)
4.9/5  (37)
(37)
For a nonmetal in Group 16 of the periodic table,the most common monatomic ion will have a charge of ____.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 41 - 59 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)