Exam 11: Introduction to Organic Molecules and Functional Groups
Exam 1: Matter and Measurement87 Questions
Exam 2: Atoms and the Periodic Table95 Questions
Exam 3: Ionic Compounds100 Questions
Exam 4: Covalent Compounds101 Questions
Exam 5: Chemical Reactions98 Questions
Exam 6: Energy Changes,reaction Rates,and Equilibrium102 Questions
Exam 7: Gases,liquids,and Solids98 Questions
Exam 8: Solutions98 Questions
Exam 9: Acids and Bases108 Questions
Exam 10: Nuclear Chemistry93 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups123 Questions
Exam 12: Alkanes104 Questions
Exam 13: Unsaturated Hydrocarbons104 Questions
Exam 14: Organic Compounds That Contain Oxygen,halogen,or Sulfur112 Questions
Exam 15: The Three-Dimensional Shape of Molecules101 Questions
Exam 16: Aldehydes and Ketones114 Questions
Exam 17: Carboxylic Acids,esters,and Amides107 Questions
Exam 18: Amines and Neurotransmitters115 Questions
Exam 19: Lipids115 Questions
Exam 20: Carbohydrates100 Questions
Exam 21: Amino Acids,proteins,and Enzymes98 Questions
Exam 22: Nucleic Acids and Protein Synthesis98 Questions
Exam 23: Metabolism and Energy Production102 Questions
Exam 24: Carbohydrate,lipid,and Protein Metabolism99 Questions
Select questions type
What is the shape about the O atoms in the environmental toxin dioxin,shown below? 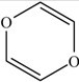
(Multiple Choice)
4.9/5  (35)
(35)
According to the common bonding patters for atoms,halogen atoms typically have ________ lone pairs of electrons when they are bonded in organic compounds.
(Multiple Choice)
4.9/5  (38)
(38)
How many total H atoms are present in the structure shown below? 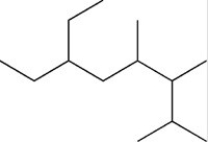
(Multiple Choice)
4.8/5  (40)
(40)
The compound whose skeletal structure is shown below is used medicinally to treat fibromyalgia pain. Which statement is NOT a valid interpretation of its skeletal structure? 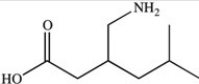
(Multiple Choice)
4.8/5  (37)
(37)
The compound below is classified as what type of compound? 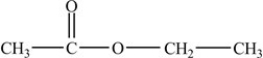
(Multiple Choice)
4.8/5  (38)
(38)
Which structure has all of the hydrogen atoms correctly added to the compound shown below? 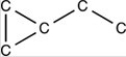
(Multiple Choice)
4.8/5  (38)
(38)
Organic compounds are produced only by living systems,and cannot be synthesized in the laboratory.
(True/False)
4.9/5  (39)
(39)
How many lone pairs of electrons are present but not shown in the molecule below? 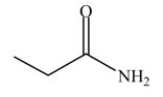
(Multiple Choice)
4.8/5  (39)
(39)
What is the correct bond angle for the bond indicated in the following structure? 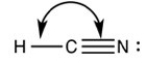
(Multiple Choice)
4.8/5  (40)
(40)
VSEPR theory is based on the concept that the most stable arrangement of atoms in a molecule keeps the atoms and lone pair electrons as far away from each other as possible.
(True/False)
4.9/5  (34)
(34)
In order to complete the structure below,________ H atom(s)and ________ lone pair(s)of electrons must be added. 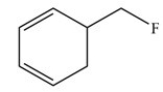
(Short Answer)
5.0/5  (44)
(44)
The molecule with the condensed formula (CH3)2CHCH2CHO can be represented as the skeletal structure below. 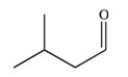
(True/False)
5.0/5  (39)
(39)
How many hydrogen atoms are bonded to the labeled carbons in the structure below? 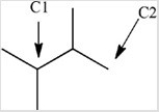
(Multiple Choice)
4.9/5  (35)
(35)
Showing 101 - 120 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)