Exam 11: Introduction to Organic Molecules and Functional Groups
Exam 1: Matter and Measurement87 Questions
Exam 2: Atoms and the Periodic Table95 Questions
Exam 3: Ionic Compounds100 Questions
Exam 4: Covalent Compounds101 Questions
Exam 5: Chemical Reactions98 Questions
Exam 6: Energy Changes,reaction Rates,and Equilibrium102 Questions
Exam 7: Gases,liquids,and Solids98 Questions
Exam 8: Solutions98 Questions
Exam 9: Acids and Bases108 Questions
Exam 10: Nuclear Chemistry93 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups123 Questions
Exam 12: Alkanes104 Questions
Exam 13: Unsaturated Hydrocarbons104 Questions
Exam 14: Organic Compounds That Contain Oxygen,halogen,or Sulfur112 Questions
Exam 15: The Three-Dimensional Shape of Molecules101 Questions
Exam 16: Aldehydes and Ketones114 Questions
Exam 17: Carboxylic Acids,esters,and Amides107 Questions
Exam 18: Amines and Neurotransmitters115 Questions
Exam 19: Lipids115 Questions
Exam 20: Carbohydrates100 Questions
Exam 21: Amino Acids,proteins,and Enzymes98 Questions
Exam 22: Nucleic Acids and Protein Synthesis98 Questions
Exam 23: Metabolism and Energy Production102 Questions
Exam 24: Carbohydrate,lipid,and Protein Metabolism99 Questions
Select questions type
In most polar bonds found in organic compounds between carbon and heteroatoms,the carbon atom has a partial ________ charge.
(Short Answer)
4.9/5  (37)
(37)
According to the common bonding patters for atoms in organic compounds,oxygen atoms typically form ________ bond(s).
(Multiple Choice)
4.7/5  (40)
(40)
Alkanes,which have no functional groups,and therefore no reactive sites,are notoriously unreactive except under very drastic conditions.
(True/False)
4.8/5  (41)
(41)
Which functional group is NOT present in the molecule below? 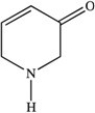
(Multiple Choice)
4.8/5  (41)
(41)
Many organic compounds contain the carbonyl group as part of their functional group. A carbonyl group has the general structure indicated below. 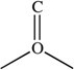
(True/False)
4.9/5  (45)
(45)
The functional group of which type of compound does not contain any multiple bonds?
(Multiple Choice)
4.8/5  (39)
(39)
Molecules with a linear shape have a bond angle of ________ around the central atom.
(Short Answer)
4.8/5  (45)
(45)
Three of the four structures below represent unstable organic compounds that are not likely to exist because they violate the octet rule. Which one of the four structures represents a stable organic compound that is likely to exist?
(Multiple Choice)
4.7/5  (34)
(34)
In order to complete the structure below,________ H atom(s)electrons must be added. 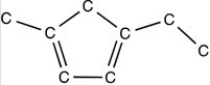
(Short Answer)
4.9/5  (46)
(46)
Showing 61 - 80 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)