Exam 9: Molecular Geometry: Shape Determines Function
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Of the following molecules (O3, SCl2, SO2, CS2, and CO2), which ones do you predict to have a bond angle of 180°?
(Short Answer)
4.8/5  (40)
(40)
Both ethane (C2H6) and ethylene (C2H4) have two carbon atoms. In a valence bond picture of the C-C bonds, ________ hybrid orbitals overlap for ethane and ________ hybrid orbitals overlap for ethylene.
(Multiple Choice)
4.8/5  (36)
(36)
Which electron-pair geometry corresponds to a steric number of 2?
(Multiple Choice)
5.0/5  (34)
(34)
What are the hybridizations of the carbon atoms in CH3CH2(CO)H, in order from left to right? Note that the 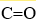 bond is a double bond.
bond is a double bond.
(Multiple Choice)
4.8/5  (33)
(33)
Which type of molecular orbital has only one nodal plane, which contains the atomic nuclei?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following statements about bonds between two carbon atoms is/are correct?
(I) Bond strength increases as more electrons are shared between the atoms.
(II) Bond strength increases as the overlap between atomic orbitals increases.
(III) Hybrid orbitals are used to describe (account for) the geometry (bond angles) around each carbon atom.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following molecules or ions has a trigonal pyramid shape?
(Multiple Choice)
4.9/5  (39)
(39)
Which electron-pair geometry has the lowest electron-electron repulsive forces?
(Multiple Choice)
4.8/5  (45)
(45)
For the molecule CH3CHCHCH3, the local molecular geometry around a carbon atom at the end and its hybridization are ________
(Multiple Choice)
4.8/5  (38)
(38)
The local molecular geometry around carbon atom 2 (second from the left) and its hybridization are ________
(Multiple Choice)
4.8/5  (37)
(37)
Identify the molecular geometry of the molecular anion, SF5-, and the hybridization of the S atomic orbitals.
(Short Answer)
4.8/5  (45)
(45)
In 1995 a Japanese cult attacked the Tokyo subway system with the nerve gas sarin, which focused world attention on the dangers of chemical warfare agents. The connectivity of atoms in the sarin molecule is shown below. Complete the Lewis structure by adding bonds and lone pairs as necessary. Assign formal charges to the P and O atoms, and determine the local molecular geometry around the central oxygen atom. 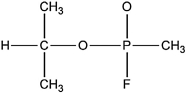
(Essay)
4.7/5  (38)
(38)
Which of the following has a local molecular geometry about a carbon atom that is trigonal planar?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following compounds has the same geometry as SO2?
(Multiple Choice)
4.7/5  (33)
(33)
Which statement about sigma ( ) and pi ( ) bonds is not correct?
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following molecules has a bond order of 3?
(Multiple Choice)
4.8/5  (38)
(38)
Which type of molecular orbital has maximum electron density above and below the internuclear axis but zero density in a plane perpendicular to the internuclear axis?
(Multiple Choice)
4.9/5  (37)
(37)
What hybridization is needed to describe the square planar molecular geometry of KrF4?
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the statements A-D about electron-pair geometry and molecular geometry is not correct?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 41 - 60 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)