Exam 9: Molecular Geometry: Shape Determines Function
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO+, and NO-? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 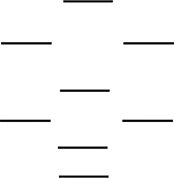
(Multiple Choice)
4.8/5  (28)
(28)
Which statement A-D about sigma ( ) and pi ( ) bonds is not correct?
(Multiple Choice)
4.9/5  (39)
(39)
Use the relative energies of the molecular orbitals given below, which are derived from the n = 5 atomic orbitals, to determine the bond orders in I2 ,
, and
, and identify the species that is predicted to be the most stable. The valence molecular orbitals in order of increasing energy are
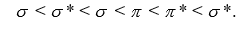
(Essay)
4.9/5  (40)
(40)
Arrange the interactions between pairs of electrons in order of increasing strength.
(Multiple Choice)
4.7/5  (31)
(31)
Identify the molecular geometry of the nitrate ion,
, and the hybridization of the N atomic orbitals.
(Short Answer)
4.8/5  (30)
(30)
Carbonyl dihalides (COX2 with X = I, Cl, or Br) are irritants and can cause blistering of tissue. Their reactivity with tissue is influenced by their polarity. Place these compounds in order of decreasing polarity and explain your reasoning.
(Essay)
4.9/5  (36)
(36)
What is the valence electron molecular orbital electron configuration of O2?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following has a central atom with the same hybridization as the oxygen in water?
(Multiple Choice)
4.8/5  (45)
(45)
Boron nitride, BN, is a new high-tech material. According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about boron nitride? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 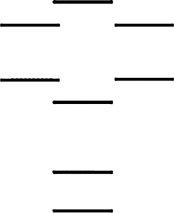
(Multiple Choice)
4.9/5  (38)
(38)
Which electron-pair geometry corresponds to a steric number of 5?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following molecules has a bond order that is less than one?
(Multiple Choice)
4.9/5  (31)
(31)
Methyl thiocyanate (CH3SCN) is used as an agricultural pesticide and fumigant. Use formal charge to identify the most stable Lewis structure for this compound. Based on this structure, predict the local molecular geometry and hybridization at each atom except hydrogen.
(Essay)
4.7/5  (31)
(31)
Ritalin is used to control attention deficit hyperactivity. Thalidomide was used in the 1950s to treat morning sickness. One enantiomer of thalidomide was found to produce birth defects. How many chiral centers are there together in these two molecules? Note hydrogen atoms attached to carbon are implicit. 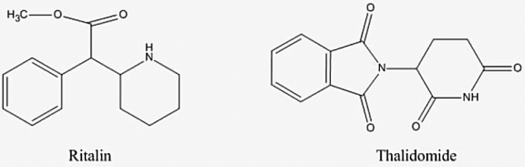
(Multiple Choice)
4.9/5  (34)
(34)
Which statement regarding a pi bond between two carbon atoms is correct?
(Multiple Choice)
4.8/5  (39)
(39)
What is the hybridization of the central iodine atom in I3-?
(Multiple Choice)
4.7/5  (38)
(38)
Showing 101 - 120 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)