Exam 9: Acids and Bases
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
The concentration of H3O+ in a solution is 1 104 M. Which of the following statements describes how the concentration of -OH in the solution could be determined?
(Multiple Choice)
5.0/5  (35)
(35)
An alkene has a pKa of 40, while an alkyne has a pKa of 25. Which functional group is more acidic?
(Multiple Choice)
4.8/5  (35)
(35)
According to the chart below, how are protons be excreted from the body? 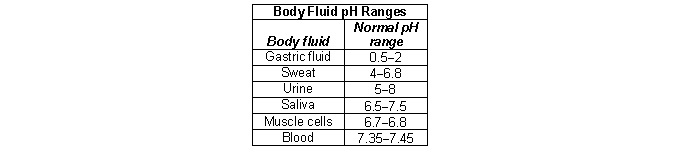
(Multiple Choice)
4.9/5  (40)
(40)
What is the pH of a solution with a [H3O+] of 7.9 1011 M?
(Multiple Choice)
4.9/5  (43)
(43)
The [H3O+] of a solution with a pH of 2 is ______ the [H3O+] of a solution with a pH of 3.
(Multiple Choice)
4.9/5  (46)
(46)
Which of the following statements does NOT correctly describe pH?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following reactions illustrate the reaction of a base? I. NH3 + H2O → +NH4 + OH
II) HCl + H2O → H3O+ + Cl
III) NaOH → Na+ + OH
IV) +NH4 + H2O → H3O+ + NH3
(Multiple Choice)
4.8/5  (26)
(26)
According to the following table, what is the most acidic fluid in the body? 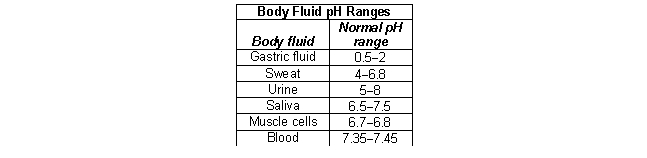
(Multiple Choice)
4.8/5  (42)
(42)
Which statement does NOT correctly explain why it is so important for intracellular pH to be maintained between 7.35 and 7.45?
(Multiple Choice)
5.0/5  (30)
(30)
Which of the following acidic functional groups is often involved in biochemical reactions in the body?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 101 - 112 of 112
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)