Exam 9: Acids and Bases
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Each circle is a sample of an aqueous acidic or basic solution. Which of the solutions contains a weak acid?
(Multiple Choice)
4.8/5  (46)
(46)
The neutralization reaction of potassium hydrogen carbonate (KHCO3) and HI produces what spectator ion?
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following types of molecules and ions is NOT a base?
(Multiple Choice)
4.8/5  (40)
(40)
When a base is dissolved in water, it reacts to give _________ and ________.
(Multiple Choice)
4.9/5  (46)
(46)
Which of the following statements best describes what happens when an acid reacts with water? I. The acid donates a proton.
II. Water donates a proton.
III. Water acts as a base.
IV. Hydronium is formed.
V. Hydroxide is formed.
(Multiple Choice)
5.0/5  (39)
(39)
The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. According to this figure, what happens when H3O+ is added to the HF/F buffer? 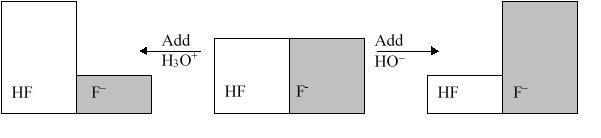
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following conditions could cause respiratory acidosis?
(Multiple Choice)
4.9/5  (36)
(36)
Water can react as both an acid and a base, depending on its environment. Because of this characteristic, water is a(n) _________ molecule.
(Multiple Choice)
4.7/5  (47)
(47)
In the acid-base reaction between ammonia and water, which bond is broken in order to give ammonium and hydroxide? 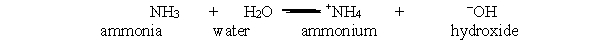
(Multiple Choice)
4.7/5  (34)
(34)
What is the pH of a solution with a [OH ] of 1.0 1010 M?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the statements best describes the following reaction? HCOOH + H2O  HCOO + H3O+
HCOO + H3O+
(Multiple Choice)
4.8/5  (33)
(33)
What is the pH of a urine sample with a hydronium concentration of 7.9 108?
(Multiple Choice)
4.8/5  (42)
(42)
Lidocaine, a common injectable dental anesthetic, is available as a 1% lidocaine-HCl solution, as shown on the following drug label. Lidocaine-HCl is a(n) 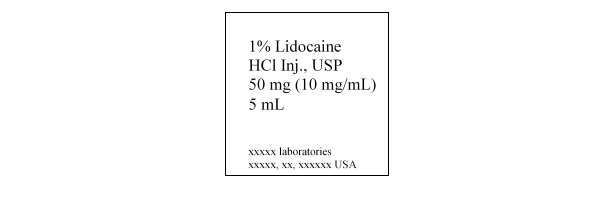
(Multiple Choice)
4.7/5  (46)
(46)
In the body, ethylene glycol (antifreeze) is metabolized to a(n)
(Multiple Choice)
4.9/5  (45)
(45)
The neutralization reaction of potassium hydrogen carbonate (KHCO3) and HI produces what gas?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following reactions best illustrates the reaction of an acid in aqueous solution?
(Multiple Choice)
5.0/5  (43)
(43)
Which of the following is a balanced equation for a neutralization reaction?
(Multiple Choice)
4.9/5  (29)
(29)
When a person hyperventilates, they breathe very quickly, exhaling CO2 more quickly than it can be produced. This reduces the concentration of CO2 in the blood. According to the chemical equation below and LeChatelier's principle, how is the bicarbonate equilibrium affected by the reduction of CO2 in the blood? 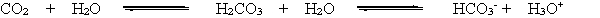
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 112
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)