Exam 9: Acids and Bases
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Lidocaine, a common injectable dental anesthetic, is available as a 1% lidocaine-HCl solution. The last step in the manufacture of lidocaine is a neutralization reaction. Which of the following reactions is the neutralization of lidocaine? 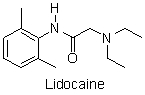
(Multiple Choice)
4.8/5  (36)
(36)
Arsenic poisoning is a serious problem in many parts of the world. When arsenic poisoning occurs, arsenic binds to proteins and eventually causes cellular damage. This leads to a variety of symptoms in humans including exhaustion, muscle weakness, organ failure, and cancer. Arsenic poisoning is commonly treated with a drug called dimercaprol (or BAL) that binds arsenic, which sets up a competing equilibrium within the body. Once arsenic reacts to form a complex with BAL, it can be excreted from the body. Arsenic-protein complex  Arsenic + proteins + BAL
Arsenic + proteins + BAL  Arsenic-BAL complex
How does treatment with BAL affect the equilibrium shown above?
Arsenic-BAL complex
How does treatment with BAL affect the equilibrium shown above?
(Multiple Choice)
4.8/5  (34)
(34)
What is the pH of a solution with a [OH ] of 4.1 103 M?
(Multiple Choice)
4.8/5  (49)
(49)
Which of the following pH ranges is referred to as physiological pH?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following are conjugate acid-base pairs in the acid-base reaction between dopamine and water?  I. H2O and
I. H2O and 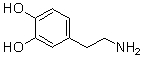 II. H2O and -OH III.
II. H2O and -OH III. 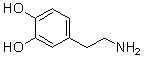 and
and 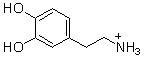 IV.
IV. 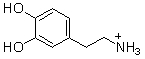 and -OH
and -OH
(Multiple Choice)
4.9/5  (41)
(41)
What is the [OH] in a solution that has a [H3O+] of 1 106 M?
(Multiple Choice)
4.8/5  (38)
(38)
Each circle is a sample of an aqueous acidic or basic solution. Which of the solutions contains a strong base?
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following buffer systems is the primary buffering system in the blood?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following solutions has the highest concentration of [OH]?
(Multiple Choice)
4.9/5  (39)
(39)
Each circle is a sample of an aqueous acidic or basic solution. Which of the solutions contains a weak base?
(Multiple Choice)
4.8/5  (39)
(39)
Consider a buffer solution containing CH3COONa+ and CH3COOH. If you add hydronium (H3O+) until all of the CH3COO is converted into CH3COOH and then add a little more hydronium, what do you expect to observe?
(Multiple Choice)
4.8/5  (47)
(47)
Adding an acid to pure water will change which of the following values: [H3O+], [HO], and/or Kw?
(Multiple Choice)
4.8/5  (40)
(40)
The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. If you add hydronium until all of the F is converted into HF and then add a little more hydronium, what is observed? 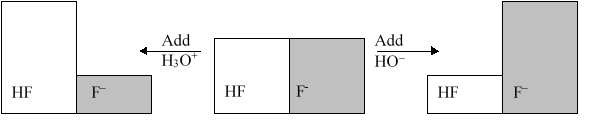
(Multiple Choice)
4.9/5  (44)
(44)
Lidocaine, a common injectable dental anesthetic, is available as a 1% lidocaine-HCl solution, as shown on the following drug label. What is the purpose of HCl in the injection solution? 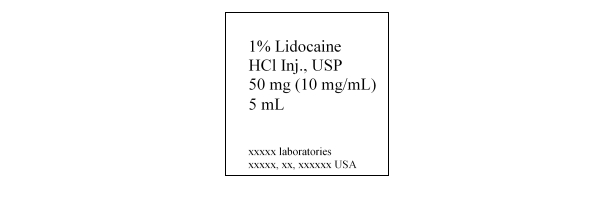
(Multiple Choice)
4.9/5  (41)
(41)
In the acid-base reaction between ammonia and water, which bond is made in order to make ammonia and water from ammonium and hydroxide? 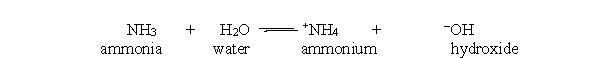
(Multiple Choice)
4.7/5  (34)
(34)
The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. What will happen when a small amount of base (OH) is added to the HF/F buffer? 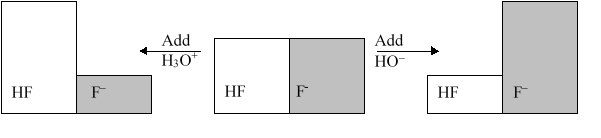
(Multiple Choice)
4.9/5  (41)
(41)
Which species in the following neutralization reaction are spectator ions? NaOH + HCl → H2O + NaCl
(Multiple Choice)
4.8/5  (36)
(36)
Showing 41 - 60 of 112
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)