Exam 9: Acids and Bases
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Which of the following changes in blood pH would you expect to observe in a hyperventilating patient, and what are those changes called?
(Multiple Choice)
4.9/5  (30)
(30)
Select the choice that correctly states whether the substance is an acid or a base.
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following would be the best treatment of metabolic acidosis?
(Multiple Choice)
4.8/5  (43)
(43)
Not all protons in a molecule can be donated in an acid-base reaction. Below are three molecules, each with the proton donated in an acid-base reaction circled. What do these protons have in common? 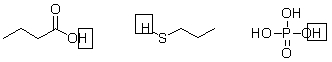
(Multiple Choice)
4.9/5  (29)
(29)
The reaction between acetic acid and water is given below, followed by a list of changes that could be made to the reaction solution. Which of these changes will result in the equilibrium shifting to the left?  Changes that could be made to the solution I. adding more CH3COOH
II) removing H2O
III) removing H3O+
IV) adding more CH3COO
Changes that could be made to the solution I. adding more CH3COOH
II) removing H2O
III) removing H3O+
IV) adding more CH3COO
(Multiple Choice)
4.8/5  (38)
(38)
What is the [H3O+] in a solution that has a [OH] of 3.2 104 M?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following compounds is a Brønsted Lowry base, but not an Arrhenius base?
(Multiple Choice)
5.0/5  (38)
(38)
All acid-base reactions that we consider in this chapter occur in
(Multiple Choice)
4.9/5  (37)
(37)
In the acid-base reaction between ammonia and water, which of the following substances are present at equilibrium? 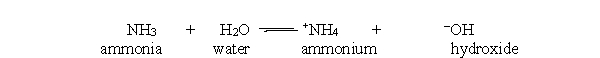
(Multiple Choice)
4.7/5  (36)
(36)
Which of the statements best describes the following reaction? 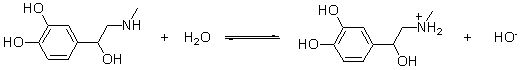
(Multiple Choice)
4.8/5  (38)
(38)
Metabolic acidosis is a type of acid-base imbalance that results in a low blood pH. It can lead to a range of symptoms including headaches, confusion, lethargy, and even death due to heart failure when the blood pH drops below 7.0. In which of the following circumstances would you suspect metabolic acidosis? 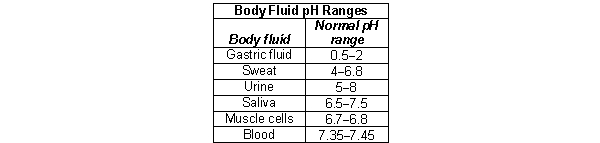
(Multiple Choice)
4.8/5  (28)
(28)
The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. Which of the following chemical equations represents the reaction that occurs when -OH is added to the HF/F buffer? 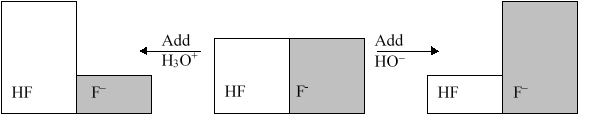
(Multiple Choice)
4.7/5  (42)
(42)
At physiological pH, phosphate esters, such as the molecule of methyl phosphate shown below, are ionized. Which of the following choices is the ionized form of a phosphate ester?
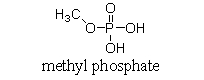
(Multiple Choice)
4.9/5  (42)
(42)
Showing 81 - 100 of 112
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)