Exam 2: Atomic Structure and Nuclear Radiation
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
_____ are the subatomic particles that have the smallest mass.
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following statements describes how our model of the atom has changed?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following describes a benefit of using rems to measure the quantity of radiation that a patient has received instead of curies?
(Multiple Choice)
4.9/5  (34)
(34)
An element is a solid at room temperature and a shiny, metallic grey. However, it is a poor conductor of electricity and temperature and it is also brittle. Which of the following elements fits this description?
(Multiple Choice)
4.7/5  (35)
(35)
According to the graph, radioactive decay is a(n) _________ in radioactivity as a function of time. 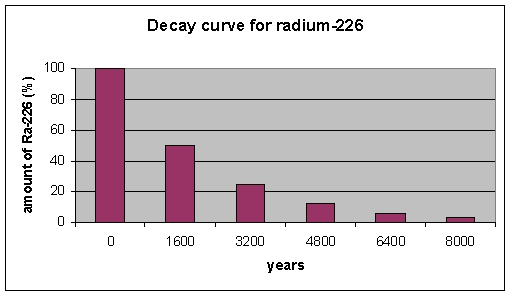
(Multiple Choice)
4.9/5  (32)
(32)
According to the current model of the atom, the part of the diagram labeled B is made up of 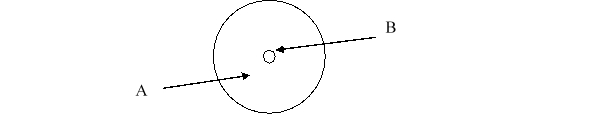
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following is NOT true for the atoms 12C, 13C, and 14C?
(Multiple Choice)
4.7/5  (41)
(41)
The energy diagram below is for an ion of magnesium. To make this ion, the atom that it came from had to_________. 
(Multiple Choice)
4.9/5  (34)
(34)
According to the periodic table, the atomic mass of potassium (K) is ______. ?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following is the best definition of the term "ionizing radiation"?
(Multiple Choice)
4.8/5  (28)
(28)
In which of the following reactions is the missing particle an alpha particle?
(Multiple Choice)
4.9/5  (41)
(41)
Which electron shell is closest to the nucleus of the atom? 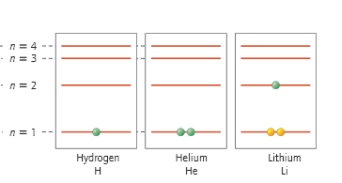
(Multiple Choice)
4.8/5  (34)
(34)
Phosphorous-32 is a beta emitter with a half-life of 14.3 days. So, after 42 days, a 100-mg sample will have decayed to 25 mg. Which statement best describes where the rest of the 32P went?
(Multiple Choice)
4.7/5  (29)
(29)
Radioisotopes used in medicine typically have short half-lives. Which of the following statements best describes the reason for this?
(Multiple Choice)
4.8/5  (45)
(45)
Electron shells closest to the nucleus are occupied before electron shells farther from the nucleus because the electrons shells closest to the nucleus are
(Multiple Choice)
4.9/5  (34)
(34)
What type of radiation is emitted when U-235 undergoes radioactive decay? 
(Multiple Choice)
4.9/5  (31)
(31)
Showing 21 - 40 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)