Exam 2: Atomic Structure and Nuclear Radiation
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
During a CT scan of the head and body, a patient receives 100 mrad of gamma radiation. What is the effective dose of gamma radiation that the patient received? Note that the quality factor for gamma radiation is 1.
(Multiple Choice)
4.8/5  (30)
(30)
Which pair does NOT correctly match an element symbol to its full name?
(Multiple Choice)
4.8/5  (34)
(34)
According to the periodic table, the atomic number of potassium (K) is ______. ?
(Multiple Choice)
4.9/5  (25)
(25)
An atom of carbon containing 7 neutrons can be written which of the following ways? 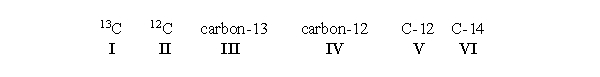 All of the choices are correct.
All of the choices are correct.
(Multiple Choice)
4.8/5  (44)
(44)
Which of the method(s) of imaging listed below produces three-dimensional images? I. x-ray
II) magnetic resonance imaging
III) computed tomography
(Multiple Choice)
4.9/5  (39)
(39)
According the graph, what percent of radium-226 remains after three half-lives? 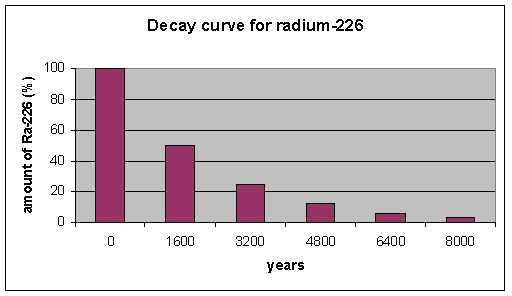
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following statement best describes why alpha particles are not frequently used in medical applications?
(Multiple Choice)
4.7/5  (30)
(30)
According to the periodic table, how many valence electrons do the elements in the third row have?
(Multiple Choice)
4.9/5  (36)
(36)
In a balanced nuclear reaction, which of the following is consistent with the release of a beta particle? ?
(Multiple Choice)
4.9/5  (26)
(26)
The conventional way of writing the symbol for an ion of calcium is
(Multiple Choice)
4.7/5  (36)
(36)
Select the choice in which atomic number, mass number, number of neutrons, and number of protons listed is correct for phosphorous-32. 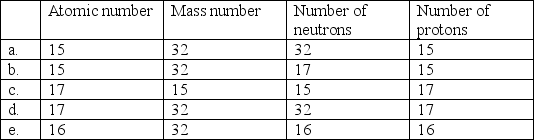
(Multiple Choice)
4.8/5  (41)
(41)
In a balanced nuclear reaction, which of the following is consistent with the release of a gamma particle??
(Multiple Choice)
4.8/5  (35)
(35)
Adding _____ to drinking water is a common practice in many cities, meant to strengthen tooth enamel and decrease dental cavities.
(Multiple Choice)
4.8/5  (36)
(36)
_____ make up the majority of compounds found in living organisms.
(Multiple Choice)
4.8/5  (40)
(40)
Which isotope in Zirconium has the fewest number of neutrons? 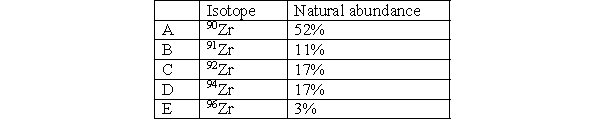
(Multiple Choice)
4.8/5  (41)
(41)
The identity of an element is determined by its number of _____.
(Multiple Choice)
4.9/5  (42)
(42)
What is the identity of the missing daughter nuclide in the following nuclear reaction? 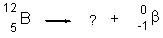
(Multiple Choice)
4.8/5  (41)
(41)
In which of the following diseases and injuries would MRI be useful in diagnosis?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 61 - 80 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)