Exam 2: Atomic Structure and Nuclear Radiation
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Which of the following does NOT need to be considered when determining what sort of protection against ionizing radiation must be used?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following is NOT the same for different isotopes of the same element?
(Multiple Choice)
4.9/5  (40)
(40)
_____ is an important component of the immune system as well as required by many enzymes.
(Multiple Choice)
4.9/5  (32)
(32)
_____ determine the physical and chemical characteristics of an atom.
(Multiple Choice)
4.9/5  (29)
(29)
How is an atom in the body changed once it is hit with ionizing radiation?
(Multiple Choice)
4.8/5  (41)
(41)
According to the periodic table, which element is in period 4, group 6A?
(Multiple Choice)
4.8/5  (34)
(34)
Elements in group 6A (16) have a 2 charge when they are in compounds. Which of the following statements best explains the reason for this observation?
(Multiple Choice)
4.8/5  (36)
(36)
Which pair correctly matches an element to its atomic number?
(Multiple Choice)
4.9/5  (27)
(27)
According to the periodic table, which of the following sets of terms accurately describes chlorine? 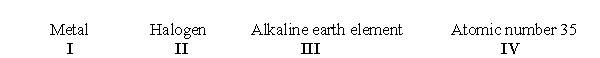
(Multiple Choice)
4.8/5  (35)
(35)
According to the periodic table, which element is found in period 2, group 5A?
(Multiple Choice)
4.7/5  (44)
(44)
According to the periodic table, what types of elements are in group 7A?
(Multiple Choice)
5.0/5  (42)
(42)
Which of the following terms is NOT a characteristic of a metal?
(Multiple Choice)
4.9/5  (37)
(37)
The energy diagram below is for an ion of magnesium. The charge on this ion is _______ making the ion a _______. 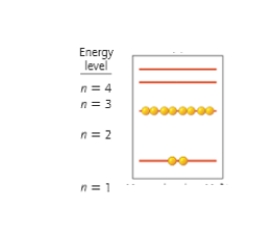

(Multiple Choice)
4.8/5  (39)
(39)
Showing 41 - 60 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)