Exam 2: Atomic Structure and Nuclear Radiation
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Which of the method(s) of imaging listed below uses x-rays? I. x-ray
II) magnetic resonance imaging
III) computed tomography
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following radioisotope would be least likely to be used in a medical application?
(Multiple Choice)
4.9/5  (47)
(47)
Which of the following statements about the model of the atom is true?
(Multiple Choice)
4.9/5  (33)
(33)
According to the periodic table, how many electron shells do the elements in the third row have?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following statements best interprets the statement below? The LD50 ?for radiation is an acute dose of 3-4 Sv.
(Multiple Choice)
4.8/5  (25)
(25)
Elements in group 1A of the periodic table are likely to form ions with the charge
(Multiple Choice)
4.8/5  (32)
(32)
Which atom has two core electrons and one valence electron? 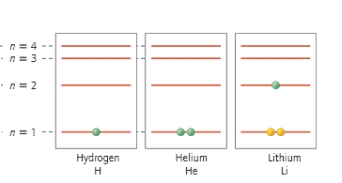
(Multiple Choice)
4.8/5  (29)
(29)
According to the periodic table, how many valence electrons do the elements in group 7A have?
(Multiple Choice)
4.8/5  (33)
(33)
In which of the following nuclear reactions is the missing particle released a beta particle?
(Multiple Choice)
4.9/5  (48)
(48)
Effective dose measurements take into account the ______ of a type of radiation.
(Multiple Choice)
4.8/5  (39)
(39)
The time that it takes a macroscopic sample of a radioisotope to decay to one-half its original activity is known as the
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following diagrams of an atom best represents the scale of the nucleus and electrons?
(Multiple Choice)
4.7/5  (34)
(34)
Showing 81 - 100 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)