Exam 18: The Solid State a Particulate View
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
If an X-ray with a wavelength of 185 pm is diffracted at an angle 2 =16.9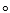 , according to the Bragg equation (n =2 d sin ), what is the distance between layers of the crystal that give rise to this diffraction? Assume n= 1 in this problem.
, according to the Bragg equation (n =2 d sin ), what is the distance between layers of the crystal that give rise to this diffraction? Assume n= 1 in this problem.
(Multiple Choice)
4.9/5  (30)
(30)
If a body-centered cubic unit cell has a volume of 1.447 *108 pm3, what must be the dimension of the cube's edge in picometers?
(Short Answer)
4.9/5  (34)
(34)
How many nearest neighbor atoms are there around each atom in a simple cubic unit cell?
(Multiple Choice)
4.9/5  (40)
(40)
In an XRD analysis using a wavelength of 154 pm, a crystalline sample of a layered VO(PO4)(H2O) structure gave peaks at 24.2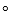 , 36.311eed92e_4ca6_1627_a0ba_7d04770a237c_TB6562_11 and 48.411eed92e_4ca6_1627_a0ba_7d04770a237c_TB6562_11.What is likely to be the value of the distance between the layers?
, 36.311eed92e_4ca6_1627_a0ba_7d04770a237c_TB6562_11 and 48.411eed92e_4ca6_1627_a0ba_7d04770a237c_TB6562_11.What is likely to be the value of the distance between the layers?
(Multiple Choice)
4.8/5  (35)
(35)
In the solid-state structure of sodium chloride, the closest distance between the centers of ions is observed
(Multiple Choice)
5.0/5  (41)
(41)
Iron crystallizes in a body-centered cubic pattern.How many iron atoms are in each unit cell?
(Multiple Choice)
4.8/5  (32)
(32)
The form of polonium (Po) crystallizes as a simple cubic unit cell with an edge length of 335 pm.What is the atomic radius of polonium in picometers?
(Short Answer)
4.7/5  (41)
(41)
Which of the following elements are found as covalent network solids?
I.beryllium, Be
II.carbon, C
III.phosphorus, P
IV.sulfur, S
(Multiple Choice)
4.9/5  (27)
(27)
Nickel (Ni) has a face-centered cubic structure with a unit cell edge length of 352.4 pm.What is the calculated value of the density of nickel in g/cm3 based on this information?
(Short Answer)
4.9/5  (31)
(31)
As an analog of graphite, a material composed of boron and nitrogen B-N) has been prepared.Why do these elements make a good substitute for the element in graphite?
I.They are all elements with electrons in the 2p subshell.
II.The sum of the valence electrons of one boron atom and one nitrogen atom is the same as the number of valence electrons on two carbon atoms.
III.Boron and nitrogen have suitable 2p orbital overlap.
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following can contain only an element of a single type?
(Multiple Choice)
4.9/5  (41)
(41)
In ionic solids, one ion often occupies a hole in the unit cell defined by the other ion.In a body-centered cubic cell, what are the types and numbers of each hole?
(Short Answer)
4.8/5  (35)
(35)
What is packing efficiency in relation to crystal structures and unit cells?
(Essay)
4.9/5  (40)
(40)
Which of the following refers to an alloy in which the composition of the elements is variable, and one element must have a much smaller radius than the other?
(Multiple Choice)
4.8/5  (47)
(47)
The diffraction pattern of an NaCl crystal is measured with 154-nm light from a copper source.Constructive interference is seen at 2 = 22.4 .What is the minimum spacing between layers giving rise to this pattern?
(Short Answer)
4.9/5  (37)
(37)
An ionic compound of iron and sulfur has a crystal structure in which sulfide ions create an fcc lattice and iron ions occupy half of the available tetrahedral holes.What is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 60 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)