Exam 18: The Solid State a Particulate View
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
According to band theory, when the lower-energy ____band is separated from the higher-energy____ band by a small band gap, the material will act as a(n) ____of electricity.
(Short Answer)
4.8/5  (36)
(36)
In the sodium chloride unit cell, the chloride ions form a cube in which each side is arranged as in the following figure.The circles represent the positions of the chloride ions on one square face of the cube.All the other faces are the same.What is the name of this unit cell? 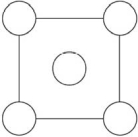
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following contributes) to the arrangement of ions in the unit cells of an ionic solid?
I.the empirical formula
II.the relative radii of the ions
III.the shape of polyatomic ions
(Multiple Choice)
4.8/5  (33)
(33)
Iron (Fe) crystallizes as a body-centered unit cell with an edge length of 287 pm.What is the atomic radius of iron?
(Multiple Choice)
4.9/5  (39)
(39)
What is the probable origin of the electrical conductivity of cadmium when its electron configuration is [Kr]4d 105s2?
(Multiple Choice)
4.9/5  (36)
(36)
When a small amount of silver is added to zinc sulfide as an activator, the band gap of the resulting material is approximately 266 kJ/mol.If this material were used as a light-emitting diode, what color of light would be emitted?
(Multiple Choice)
4.8/5  (34)
(34)
Aluminum (Al) has a density of 2.70 g/cm3 and crystallizes in a face-centered cubic structure.What is the unit cell edge length?
(Multiple Choice)
4.8/5  (30)
(30)
An unknown metal with an fcc structure has a density of 10.5 g/cm3, and the edge length of the unit cell is 409 pm.What is the probable identity of the metal?
(Multiple Choice)
4.9/5  (31)
(31)
Magnesium oxide adopts a rock salt structure with a density of approximately 3.58 g/cm3.Based on this information, calculate an estimate of the unit cell edge length in picometers.
(Multiple Choice)
4.8/5  (37)
(37)
Consider the three unit cells below for ionic solids.In each unit cell, one of the ions is arranged in one of the standard unit cell structures while the other is found between these sites.What are the names of the minerals associated with each of these structures? I _____; II_____ ; III_____ . 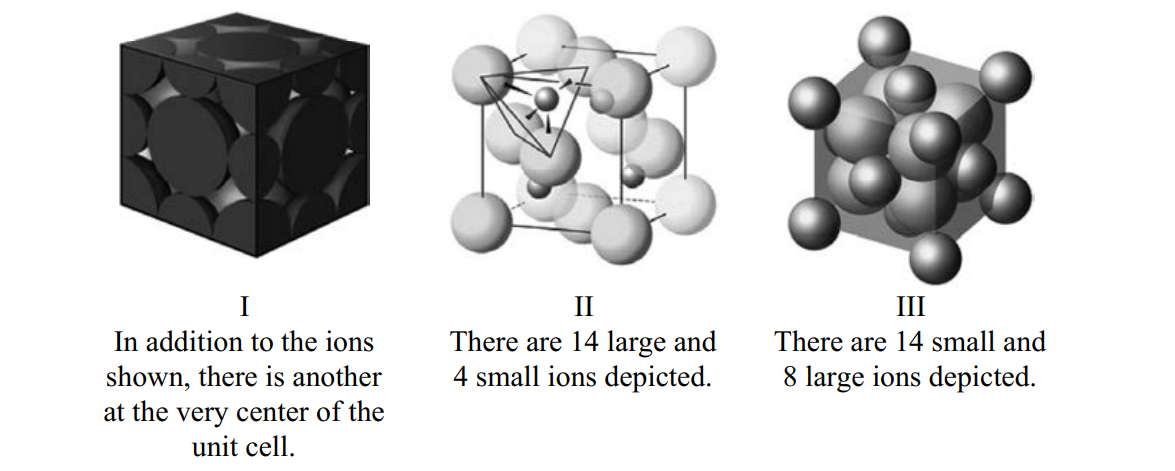
(Essay)
4.8/5  (34)
(34)
A ceramic is a chemically resistant and heat-resistant solid produced by heating
(Multiple Choice)
4.8/5  (37)
(37)
Cesium bromide adopts a cubic structure in which bromide ions lie at the corners of the cube with the cesium ions lying in the holes in the center of the cubes.The density of CsBr is 4.44 g/cm3.Calculate the approximate edge length of the unit cell in picometers.
(Short Answer)
4.9/5  (24)
(24)
How many nearest neighbor atoms are there around each atom in a body-centered cubic unit cell?
(Multiple Choice)
4.7/5  (40)
(40)
Gold (Au) has a face-centered cubic structure with a unit cell edge length of 407.8 pm.What is the calculated value of the density of gold based on this information?
(Multiple Choice)
4.8/5  (45)
(45)
Fragments of a linear allotrope of carbon were first prepared and characterized in 1998.Would you expect this form of carbon to conduct electricity?
(Multiple Choice)
4.9/5  (40)
(40)
An approximately spherical allotrope of carbon containing 60 or 70 atoms is
(Multiple Choice)
4.8/5  (40)
(40)
When germanium (Ge) is doped with gallium (Ga), it produces a(n)______ -type semiconductor.
(Multiple Choice)
4.7/5  (40)
(40)
Describe the ccp and hcp crystal structures.Include the arrangement of atoms in one layer as well as how layers are stacked to form the crystal lattice.
(Essay)
4.8/5  (39)
(39)
How many tennis balls will fit within the interstitial holes between a truckload of basketballs perfectly placed in a closest-packed arrangement? Assume that the tennis balls have a radius that is 20% that of a basketball.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 61 - 80 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)