Exam 30: Nuclear Physics and Radioactivity
Exam 1: Introduction, Measurement, Estimating29 Questions
Exam 2: Describing Motion: Kinematics in One Dimension527 Questions
Exam 3: Kinematics in Two Dimensions; Vectors183 Questions
Exam 4: Dynamics: Newtons Laws of Motion146 Questions
Exam 5: Circular Motion; Gravitation105 Questions
Exam 6: Work and Energy153 Questions
Exam 7: Linear Momentum139 Questions
Exam 8: Rotational Motion148 Questions
Exam 9: Static Equilibrium; Elasticity and Fracture83 Questions
Exam 10: Fluids98 Questions
Exam 11: Oscillations and Waves114 Questions
Exam 12: Sound21 Questions
Exam 13: Temperature and Kinetic Theory87 Questions
Exam 14: Heat88 Questions
Exam 15: The Laws of Thermodynamics78 Questions
Exam 16: Electric Charge and Electric Field99 Questions
Exam 17: Electric Potential107 Questions
Exam 18: Electric Currents96 Questions
Exam 19: Dc Circuits384 Questions
Exam 20: Magnetism164 Questions
Exam 21: Electromagnetic Induction and Faradays Law60 Questions
Exam 22: Electromagnetic Waves167 Questions
Exam 23: Light: Geometric Optics144 Questions
Exam 24: The Wave Nature of Light58 Questions
Exam 25: Optical Instruments156 Questions
Exam 26: The Special Theory of Relativity126 Questions
Exam 27: Early Quantum Theory and Models of the Atom192 Questions
Exam 28: Quantum Mechanics of Atoms74 Questions
Exam 29: Molecules and Solids26 Questions
Exam 30: Nuclear Physics and Radioactivity153 Questions
Exam 31: Nuclear Energy; Effects and Uses of Radiation36 Questions
Exam 32: Elementary Particles19 Questions
Exam 33: Astrophysics and Cosmology25 Questions
Select questions type
How much energy is released when of have decayed to ? The mass of is , the mass of is , and .
(Short Answer)
4.9/5  (28)
(28)
The number of radioactive nuclei in a particular sample decreases to one-eighth of its original number in 9 days. What is the half-life of these nuclei?
(Multiple Choice)
4.8/5  (26)
(26)
Rubidium is a naturally occurring isotope that undergoes decay. What isotope is the product of this decay?
(Multiple Choice)
4.8/5  (38)
(38)
A summary of the nuclear reactions that power our sun is , with masses of for a proton, for the helium nucleus, and for each electron. How much energy is released by each of these reactions?
(Multiple Choice)
4.8/5  (27)
(27)
A radioactive isotope of atomic number emits an alpha particle, and the daughter nucleus then emits a beta-minus particle. What is the atomic number of the resulting nucleus?
(Multiple Choice)
4.9/5  (31)
(31)
A nuclear reaction is shown: . Which one of the following isotopes is the missing nuclear product?
(Multiple Choice)
4.9/5  (34)
(34)
A radioactive sample has a half-life of . What fraction of the sample is left after ?
(Multiple Choice)
4.7/5  (34)
(34)
If the half-life of a material is 45 years, how much of it will be left after 100 years?
(Multiple Choice)
4.9/5  (36)
(36)
The neutral deuterium atom, , has a mass of ; a neutral ordinary hydrogen atom has a mass of ; a neutron has a mass of ; and a proton has a mass of . What is the binding energy of the deuterium nucleus?
(Multiple Choice)
4.9/5  (35)
(35)
The radioactivity due to carbon-14 measured in a piece of a wooden casket from an ancient burial site is found to produce 20 counts per minute from a given sample, whereas the same amount of
Carbon from a piece of living wood produces 160 counts per minute. The half-life of carbon-14, a
Beta-minus emitter, is 5730 years. What would we calculate for the age of the artifact, assuming
That the activity for living wood has remained constant over time?
(Multiple Choice)
4.7/5  (32)
(32)
Plutonium has a half life of years. How long does it take for of the plutonium to decay?
(Multiple Choice)
4.9/5  (42)
(42)
A hospital patient has been given a sample of 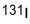 , which has a half-life of days. This sample is decaying at times the acceptable level for exposure to the general public. How long must the patient wait for the decay rate of the sample to reach the acceptable level?
, which has a half-life of days. This sample is decaying at times the acceptable level for exposure to the general public. How long must the patient wait for the decay rate of the sample to reach the acceptable level?
(Multiple Choice)
4.9/5  (28)
(28)
An old bone sample contains of carbon and has an activity of 947 decays/min due to the carbon-14 in it. The half-life of carbon-14 is . Assume that the activity of the atmospheric carbon is, and has remained, per gram of carbon.
(a) How old is the sample?
(b) What will be the activity of the sample from now, in decays per minute?
(Short Answer)
4.8/5  (36)
(36)
An excited nucleus undergoes fission into two fragments as follows:
If, at the instant of fission, the and fragments are spherical and just barely in contact, what is the electrostatic potential energy of these two fragments? ( , )
(Multiple Choice)
4.9/5  (34)
(34)
What happens to the half-life of a radioactive substance as we increase its temperature?
(Multiple Choice)
4.9/5  (33)
(33)
The following masses are known:
1.008665 1.007825 226.025403
What is the binding energy of ?
(Multiple Choice)
4.9/5  (27)
(27)
A sample of wood has been recovered by an archaeologist. The sample is sent to a laboratory, where it is determined that the activity of the sample is 0.144 Bq/g. By comparing this activity with
The activity of living organic matter, which is 0.230 Bq/g, the scientist determines how old the wood
Sample is, or more precisely, when the tree that the sample came from died. Carbon-14 has a
Half-life of 5730 years. If the activity of living matter has been constant over time, how old is the
Sample of wood?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 41 - 60 of 153
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)