Exam 30: Nuclear Physics and Radioactivity
Exam 1: Introduction, Measurement, Estimating29 Questions
Exam 2: Describing Motion: Kinematics in One Dimension527 Questions
Exam 3: Kinematics in Two Dimensions; Vectors183 Questions
Exam 4: Dynamics: Newtons Laws of Motion146 Questions
Exam 5: Circular Motion; Gravitation105 Questions
Exam 6: Work and Energy153 Questions
Exam 7: Linear Momentum139 Questions
Exam 8: Rotational Motion148 Questions
Exam 9: Static Equilibrium; Elasticity and Fracture83 Questions
Exam 10: Fluids98 Questions
Exam 11: Oscillations and Waves114 Questions
Exam 12: Sound21 Questions
Exam 13: Temperature and Kinetic Theory87 Questions
Exam 14: Heat88 Questions
Exam 15: The Laws of Thermodynamics78 Questions
Exam 16: Electric Charge and Electric Field99 Questions
Exam 17: Electric Potential107 Questions
Exam 18: Electric Currents96 Questions
Exam 19: Dc Circuits384 Questions
Exam 20: Magnetism164 Questions
Exam 21: Electromagnetic Induction and Faradays Law60 Questions
Exam 22: Electromagnetic Waves167 Questions
Exam 23: Light: Geometric Optics144 Questions
Exam 24: The Wave Nature of Light58 Questions
Exam 25: Optical Instruments156 Questions
Exam 26: The Special Theory of Relativity126 Questions
Exam 27: Early Quantum Theory and Models of the Atom192 Questions
Exam 28: Quantum Mechanics of Atoms74 Questions
Exam 29: Molecules and Solids26 Questions
Exam 30: Nuclear Physics and Radioactivity153 Questions
Exam 31: Nuclear Energy; Effects and Uses of Radiation36 Questions
Exam 32: Elementary Particles19 Questions
Exam 33: Astrophysics and Cosmology25 Questions
Select questions type
Two radioactive isotopes, X and Y, both decay to stable products. The half-life of X is about a day, while that of Y is about a week. Suppose a radioactive sample consists of a mixture of these two
Nuclides. If the mixture is such that the activities arising from X and Y are initially equal, then a few
Days later the activity of the sample will be due
(Multiple Choice)
4.8/5  (38)
(38)
One of the fusion reactions that occurs in the sun is:
The following atomic masses are known:
What is the reaction energy released in this fusion reaction?
(Multiple Choice)
4.8/5  (37)
(37)
The half-life of a radioactive material is days. How many days are required for a sample, with an initial activity of , to decay to an activity of ?
(Multiple Choice)
4.8/5  (33)
(33)
One material used in nuclear bombs is , with a half-life of 24,000 years. How long must we wait for a buried stockpile of this substance to decay to of its original activity?
(Multiple Choice)
4.9/5  (45)
(45)
Bismuth is known to be radioactive. The stability of with respect to alpha, beta-plus and beta-minus decay is to be determined. The following atomic masses are known:
: 4.002603 : 207.981998 : 211.991871 : 211.991255 : 211.988842 The Bi nucleus is
(Multiple Choice)
4.8/5  (44)
(44)
Isotope A has a decay constant of and isotope B has a decay constant of . Which isotope has a longer mean life (time constant)?
(Multiple Choice)
4.8/5  (26)
(26)
The stability of with respect to alpha, beta-plus and beta-minus decay is to be determined.
The following atomic masses are known:
The nucleus is
(Multiple Choice)
4.9/5  (46)
(46)
A proton is projected at a stationary Ra aluminum target. The proton momentarily comes to a halt at a distance from the center of an aluminum nucleus, equal to twice the nuclear radius. Assume that the nucleus retains its spherical shape and that the nuclear force on the proton is negligible. The initial kinetic energy of the proton, in , is closest to:
(Multiple Choice)
4.9/5  (35)
(35)
The unstable isotope 234Th decays by emission with a half-life of days. If the initial decay rate of the sample is , what is the decay rate after 37 days?
(Short Answer)
4.7/5  (42)
(42)
The stability of with respect to alpha, beta-plus and beta-minus decay is to be determined.
The following atomic masses are known:
The nucleus is
(Multiple Choice)
4.8/5  (39)
(39)
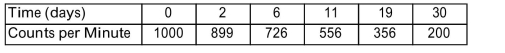 In a laboratory accident, a work area is contaminated with radioactive material. Health physicists monitor the area during a 30-day period and obtain the data shown in the table. The accident occurred at time days. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 16 counts per minute. Of the choices listed, which one is the earliest time after the accident that workers could safely return?
In a laboratory accident, a work area is contaminated with radioactive material. Health physicists monitor the area during a 30-day period and obtain the data shown in the table. The accident occurred at time days. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 16 counts per minute. Of the choices listed, which one is the earliest time after the accident that workers could safely return?
(Multiple Choice)
4.8/5  (34)
(34)
Fermium-253 has a half-life of days. If a sample of fermium originally has nuclei, how long will it take for there to be only fermium-253 nuclei left in this sample?
(Multiple Choice)
4.8/5  (37)
(37)
What are the mass number A and the charge (in units of e) for each of the following
particles or rays?
(a) beta-plus
(b) beta-minus
(c) gamma ray
(Short Answer)
4.8/5  (42)
(42)
The isotope is radioactive and decays in a series to Th. In this series, the particles ejected from the nucleus are
(Multiple Choice)
4.8/5  (35)
(35)
If an atomic nucleus containing 64 nucleons has a radius , what will be the expected radius of a nucleus containing 512 nucleons?
(Multiple Choice)
4.9/5  (30)
(30)
A radioactive source emits neutrons at the rate of 7100 neutrons per second. The number of atoms in the source is . What is the activity of the source, in curies (Ci)? )
(Multiple Choice)
4.7/5  (40)
(40)
Showing 61 - 80 of 153
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)