Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
Iron metal reacts with hydrochloric acid as follows: 2Fe(s) + 6HCl(aq) 2FeCl3(aq) + 3H2(g) If 35.6 g of iron react with excess HCl, and 98.6 g of FeCl3 are collected, what is the percent yield of FeCl3?
(Multiple Choice)
4.9/5  (42)
(42)
Aluminum metal reacts with sulfuric acid according to the following equation: 2Al(s) + 3H2SO4(aq) Al2(SO4)3(s) + 3H2(g) If 10.0 g of aluminum reacts with excess sulfuric acid, and 54.2 g of Al2(SO4)3 are collected, what is the percent yield of Al2(SO4)3?
(Multiple Choice)
4.9/5  (33)
(33)
The coefficients of a balanced equation can be understood to represent either relative numbers of moles or relative mass.
(True/False)
4.8/5  (44)
(44)
In the process of obtaining lead from PbS, or galena, the galena is "roasted" (heated in the presence of oxygen), so that the following reaction occurs: 2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g) If 50.0 g of PbS are mixed with 25.0 g of oxygen, how many grams of PbO should form?
(Multiple Choice)
4.8/5  (33)
(33)
The specific heat of a substance is the amount of heat energy that is needed to cause the substance to melt.
(True/False)
4.8/5  (33)
(33)
Nitrogen and hydrogen react together to form ammonia according to the equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen would be required to react with 50.4 g of nitrogen.
(Multiple Choice)
4.8/5  (33)
(33)
Nitrogen and hydrogen react together to form ammonia according to the equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen reactant would be required to produce 25.0 g of ammonia, assuming there is sufficient nitrogen available.
(Multiple Choice)
4.7/5  (42)
(42)
If you wish to make sandwiches which consist of two slices of bread, one ham slice, and three pickle slices, how many sandwiches could you make if you have 12 slices of bread, five slices of ham, and 20 pickle slices, and what would be left over?
(Multiple Choice)
4.9/5  (37)
(37)
Iron metal reacts with chlorine gas according to the following equation: 2Fe(s) + 3Cl2(g) 2FeCl3(s) If 35.0 g each of iron and chlorine are combined, how much FeCl3 should form?
(Multiple Choice)
4.9/5  (35)
(35)
What mass (in grams) of SF6 should be produced by the following reaction if 7.00 g of sulfur is mixed with 9.00 g of fluorine? S + 3F2 SF6
(Multiple Choice)
4.9/5  (37)
(37)
Consider the following specific heats of metals. 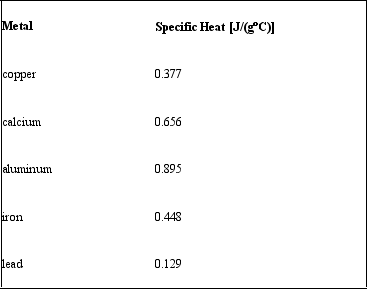 If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
(Multiple Choice)
4.9/5  (40)
(40)
Nitrogen and hydrogen react together to form ammonia according to the equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen would be required to react with 25.2 g of nitrogen.
(Multiple Choice)
4.8/5  (34)
(34)
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s) + Cl2(g) 2NaCl(s) If the mass of the sodium solid increases by 0.500 g, what mass of sodium metal should have reacted?
(Multiple Choice)
4.7/5  (40)
(40)
When sodium sulfate, Na2SO4, dissolves in water, the ions that are formed for each formula unit that dissolves are:
(Multiple Choice)
4.8/5  (37)
(37)
Consider the reaction between hydrogen and oxygen gases to form water: 2H2(g) + O2(g) 2H2O(l) If 8.5 moles of oxygen react with sufficient hydrogen, how many grams of water should form?
(Multiple Choice)
4.9/5  (38)
(38)
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the following equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of phosphorus reactant would be required to produce 75.0 g of phosphorus trichloride, assuming there is sufficient chlorine available.
(Multiple Choice)
4.9/5  (25)
(25)
When acetylene, C2H2, a fuel used in welding, is burned in air, two molecules of acetylene combine with five oxygen molecules to form four CO2 molecules and two H2O molecules.Select the statement below that is incorrect in regard to this reaction.
(Multiple Choice)
4.9/5  (44)
(44)
Consider the following reaction: Cr2O3(s) + 3CCl4(l) 2CrCl3(s) + 3COCl2(g) green colorless purple colorless solid liquid solid gas When the green solid is mixed with the colorless liquid, the mixture starts to bubble and fume.When all action has stopped, a dry purple solid containing solid green specks remains.Which substance is the limiting reactant?
(Multiple Choice)
5.0/5  (36)
(36)
All of the following may change during a chemical reaction except
(Multiple Choice)
4.9/5  (40)
(40)
The number of moles of reactant molecules must always equal the number of moles of product molecules in a balanced equation.
(True/False)
4.7/5  (35)
(35)
Showing 21 - 40 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)