Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
Given the balanced equation 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 82.0 g of NH3 react with sufficient oxygen, how many grams of NO should form?
(Multiple Choice)
4.8/5  (32)
(32)
What is the heat change when a 53.5 g sample of water [Cwater = 4.184 J/(g C)] is cooled from 98.0 C to 23.2 C?
(Multiple Choice)
4.8/5  (38)
(38)
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products.Which of the following reactions could this represent? 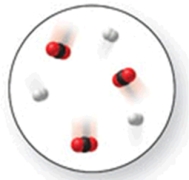
(Multiple Choice)
4.9/5  (40)
(40)
Ammonia is usually made by the following reaction: N2(g) + 3H2(g) 2NH3(g) What is the maximum amount of ammonia that can be formed if 30 molecules of nitrogen are mixed with 100 molecules of hydrogen?
(Multiple Choice)
4.7/5  (32)
(32)
Small amounts of oxygen gas can be produced for laboratory use by heating potassium chlorate, which causes it to decompose by the following reaction: ___KClO3(s) ____KCl(s) + ___O2(g) (unbalanced) Balance the equation, and determine the mass of oxygen that should be formed if 10.0 g of potassium chlorate decomposes.
(Multiple Choice)
4.9/5  (39)
(39)
Given that 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 8.2 moles of NH3 react with sufficient oxygen, how many moles of water should form?
(Multiple Choice)
4.9/5  (37)
(37)
Consider the following reaction: 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) How many moles of NO2 are required to react with 1.50 moles of H2O to form 3.00 moles of HNO3?
(Multiple Choice)
4.9/5  (39)
(39)
The following reaction releases 2800 kJ of heat for each mole of C6H12O6 that reacts. C6H12O6(s) + 6O2(g) 6CO2(g) + 6H2O(l) This reaction is
(Multiple Choice)
4.8/5  (28)
(28)
One calorie is the amount of heat energy required to raise the temperature of 10.0 g of water by 1 C.
(True/False)
4.8/5  (32)
(32)
Phosphorus trichloride can be made by the following reaction: P4(s) + 6Cl2(g) 4PCl3(l) What is the maximum amount of phosphorus trichloride that can be formed if 10 molecules of P4 are mixed with 36 molecules of chlorine?
(Multiple Choice)
4.9/5  (32)
(32)
How much heat energy would be needed to raise the temperature of a 32.0 g sample of gold [C = 0.129 (J/g C)] from 21.8 C to 75.0 C?
(Multiple Choice)
4.9/5  (35)
(35)
When 5.0 g CaCl2 is dissolved in enough water to make a 0.500 L solution, what is the molarity of ions in solution?
(Multiple Choice)
4.8/5  (36)
(36)
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products.Which of the following reactions could this represent? 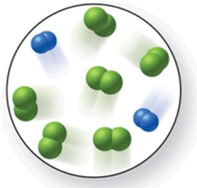
(Multiple Choice)
4.8/5  (45)
(45)
If you have eight bicycle wheels and five frames, how many bikes could you build (assuming that each bike requires one frame and two wheels), and what would be left over?
(Multiple Choice)
4.8/5  (27)
(27)
When potassium carbonate, K2CO3, dissolves in water, the ions that are formed for each formula unit that dissolves are:
(Multiple Choice)
4.9/5  (28)
(28)
When ammonium carbonate, (NH4)2CO3, dissolves in water, the ions that are formed for each formula unit that dissolves are:
(Multiple Choice)
4.8/5  (30)
(30)
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s) + Cl2(g) 2NaCl(s) If 12.5 g of sodium react with sufficient chlorine, how many grams of sodium chloride should form?
(Multiple Choice)
4.9/5  (45)
(45)
Nitrogen and hydrogen react together to form ammonia according to the following equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen reactant would be required to produce 50.0 g of ammonia, assuming there is sufficient nitrogen available.
(Multiple Choice)
4.9/5  (39)
(39)
Showing 101 - 120 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)