Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
If the theoretical yield for a reaction is 54.9 g, and 51.3 g of product are actually obtained, the percent yield is:
(Multiple Choice)
4.8/5  (34)
(34)
A can of soda has 1.50 x 102 Calories.Convert this energy to units of joules.
(Multiple Choice)
4.9/5  (34)
(34)
The coefficients of a balanced equation can be understood to represent either relative numbers of molecules or moles.
(True/False)
4.8/5  (42)
(42)
When sulfur dioxide is formed from its elements, 296.8 kJ of energy is released.Convert this energy to units of calories.
(Multiple Choice)
4.7/5  (31)
(31)
When calculating the percent yield for a reaction, the only information necessary is the mass of each reactant.
(True/False)
4.8/5  (33)
(33)
Iron metal reacts with chlorine gas according to the following equation: 2Fe(s) + 3Cl2(g) 2FeCl3(s) If 25.6 g each of iron and chlorine are combined, how much FeCl3 should form?
(Multiple Choice)
4.8/5  (40)
(40)
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) + 8O2(g) P4O10(s) + 6H2O(g) If 9.2 moles of phosphine react with sufficient oxygen, how many moles of P4O10 should form?
(Multiple Choice)
4.8/5  (27)
(27)
A 5.00 g sample of a brownie was burned in a bomb calorimeter containing 2025 g of water.The temperature of the water increased from 23.50 C to 33.47 C.How much heat, in joules, did the brownie release when it burned? [Cwater = 4.184 J/(g C)]
(Multiple Choice)
4.8/5  (45)
(45)
Aluminum metal reacts with sulfuric acid according to the following equation: 2Al(s) + 3H2SO4(aq) Al2(SO4)3(s) + 3H2(g) If 12.9 g of aluminum reacts with excess sulfuric acid, and 62.4 g of Al2(SO4)3 are collected, what is the percent yield of Al2(SO4)3?
(Multiple Choice)
4.8/5  (34)
(34)
A calorie used by nutritionists, 1 Calorie, is equal to 1000 cal or 1 kcal used by chemists.
(True/False)
5.0/5  (36)
(36)
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 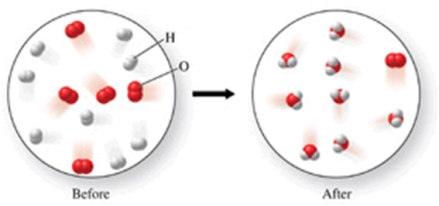
(Multiple Choice)
4.8/5  (41)
(41)
Consider the reaction N2(g) + 2O2(g) 2NO2(g).The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 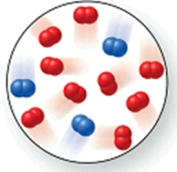
(Multiple Choice)
4.9/5  (35)
(35)
A 43 g serving of a chocolate candy has 2.10 x 102 Calories.Convert this energy to units of joules.
(Multiple Choice)
4.8/5  (39)
(39)
When fats or other foods are burned in a bomb calorimeter, the calorimeter absorbs heat, so the reaction is endothermic.
(True/False)
4.9/5  (32)
(32)
When carbon dioxide is formed from its elements, 393.5 kJ of energy is released.Convert this energy to units of calories.
(Multiple Choice)
4.8/5  (36)
(36)
An energy input of 227 kJ is required to form acetylene from its elements.Convert this energy to units of calories.
(Multiple Choice)
4.8/5  (34)
(34)
The q value for the following reaction is 178.0 kJ for every mole of CaCO3 that reacts: CaCO3(s) CaO(s) + CO2(g) How much heat would be required to decompose 4.00 mol CaCO3(s), and is the reaction endothermic or exothermic?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 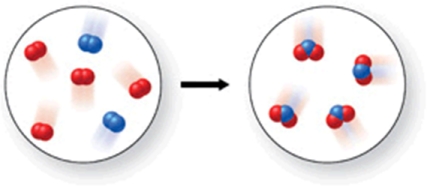
(Multiple Choice)
4.8/5  (34)
(34)
Showing 81 - 100 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)