Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
How much heat energy would be needed to raise the temperature of a 15.0 g sample of iron [C = 0.448 J/(g C)] from 22.0 C to 100.0 C?
(Multiple Choice)
4.9/5  (35)
(35)
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s) + Cl2(g) 2NaCl(s) If 3.6 moles of chlorine react with sufficient sodium, how many grams of sodium chloride should form?
(Multiple Choice)
4.8/5  (35)
(35)
Aluminum reacts with oxygen according to the following reaction: 4Al(s) + 3O2(g) 2Al2O3(s) If 12 moles of aluminum are combined with 6 moles of oxygen, how many moles of Al2O3 should form?
(Multiple Choice)
4.8/5  (37)
(37)
How much heat energy would be needed to raise the temperature of a 22.3 g sample of aluminum [(C = 0.895 (J/g C)] from 22.5 C to 55.0 C?
(Multiple Choice)
4.7/5  (32)
(32)
Equal masses of ice at 0 C and water at 100 C are mixed in an insulated container.Estimate the final temperature of the mixture.
(Multiple Choice)
4.8/5  (37)
(37)
Consider the following reaction: 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) How many moles of the excess reactant remain if 4.00 moles of H2O and 10.00 moles of NO2 are mixed?
(Multiple Choice)
4.8/5  (41)
(41)
When mixed, solutions of aluminum nitrate, Al(NO3)3, and ammonium carbonate, (NH4)2CO3, will form a precipitate of aluminum carbonate, Al2(CO3)3.The balanced equation is 2Al(NO3)3(aq) + 3(NH4)2CO3(aq) Al2(CO3)3 (s) + 6NH4NO3(aq) Which of the following statements regarding this reaction is incorrect?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following best describes an exothermic reaction?
(Multiple Choice)
5.0/5  (31)
(31)
When 3.0 mol CaCl2 dissolves in water, how many moles of ions are in solution?
(Multiple Choice)
4.8/5  (36)
(36)
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of phosphorus reactant would be required to produce 25.0 g of phosphorus trichloride, assuming there is sufficient chlorine available.
(Multiple Choice)
4.9/5  (40)
(40)
If 75.0 J of heat energy is added to 25.0 g samples of different metals.Given their specific heat values, rank the metals in order from least to greatest final temperature. 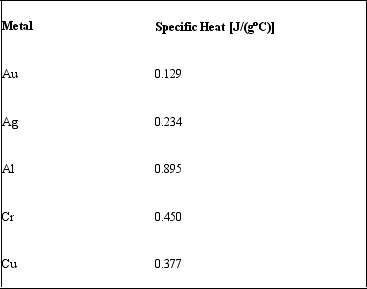
(Multiple Choice)
4.8/5  (35)
(35)
When a 0.525 g piece of zinc is placed in a solution of copper(II) sulfate, copper metal and zinc sulfate are formed.Balance the equation for the reaction, and determine the mass of copper(II) sulfate that would react with this quantity of zinc. ___ Zn(s) + ___CuSO4(aq) ___ZnSO4(aq) + ___Cu(s) (unbalanced)
(Multiple Choice)
4.7/5  (37)
(37)
The figure shows a molecular-level diagram of reactant molecules for the reaction 2H2(g) + O2(g) 2H2O(l) 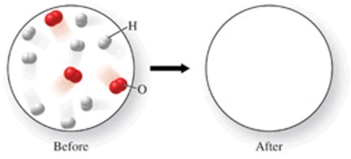 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
(Multiple Choice)
4.7/5  (32)
(32)
A pamphlet requires one cover, 14 pieces of white paper, and three sheets of colored paper.How many pamphlets could be made, and what would be left over, if there are 50 covers, 500 sheets of white paper, and 100 sheets of colored paper available?
(Multiple Choice)
5.0/5  (33)
(33)
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) + 8O2(g) P4O10(s) + 6H2O(g) If you need to make 6.5 moles of P4O10 , how many moles of PH3 is required for the reaction?
(Multiple Choice)
4.7/5  (39)
(39)
Ammonia is usually made by the following reaction: N2(g) + 3H2(g) 2NH3(g) What is the maximum amount of ammonia that can be formed if 25 molecules of nitrogen are mixed with 60 molecules of hydrogen?
(Multiple Choice)
4.8/5  (43)
(43)
When mercury(II) oxide, a red crystalline solid, is heated, it decomposes to form liquid mercury and oxygen gas, according to the following equation: ___HgO(s) ___Hg(l) + ___O2(g) (unbalanced) Balance the equation and determine the mass of mercury that should be formed when 12.3 g of HgO is heated.
(Multiple Choice)
4.9/5  (38)
(38)
Given that 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 6.3 moles of NH3 react with sufficient oxygen, how many moles of NO should form?
(Multiple Choice)
4.9/5  (34)
(34)
A carton of low-fat yogurt says it has 1.70 x 102 Calories.What is the equivalent amount of energy in units of joules?
(Multiple Choice)
4.8/5  (29)
(29)
Showing 61 - 80 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)