Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
An equal quantity of heat is transferred to 10.0 g samples of different substances.Given their specific heat values, rank the substances in order from least to greatest final temperature. 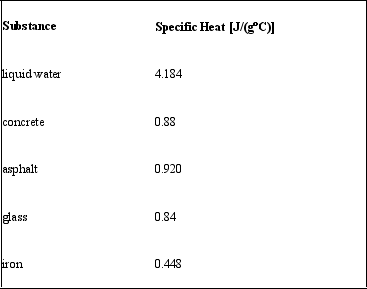
(Multiple Choice)
4.8/5  (41)
(41)
When a substance cools from a high temperature to a low temperature, its heat change value will have a negative sign.
(True/False)
4.8/5  (37)
(37)
Consider the following reaction: CrCl3(s) + KCl(s) + 2H2SO4(l) KCr(SO4)2(s) + 4HCl(g) green white colorless purple colorless solid solid liquid solid gas When the green solid is mixed with the white solid and the colorless liquid is added, the mixture starts to bubble and fume.When all action has stopped, a wet purple solid containing solid white specks remains.Which substance is the limiting reactant?
(Multiple Choice)
4.9/5  (42)
(42)
What is the heat change when a 225 g sample of olive oil [Cwater = 1.79 J/(g C)] is cooled from 95.8 C to 52.1 C?
(Multiple Choice)
4.9/5  (42)
(42)
Phosphorus trichloride can be made by the following reaction: P4(s) + 6Cl2(g) 4PCl3(l) What is the maximum amount of phosphorus trichloride that can be formed if 15 molecules of P4 are mixed with 42 molecules of chlorine?
(Multiple Choice)
4.8/5  (43)
(43)
Consider the reaction between sodium metal and chlorine gas to form sodium chloride: 2Na(s) + Cl2(g) 2NaCl(s) Which of the following is not conserved in this reaction?
(Multiple Choice)
4.8/5  (35)
(35)
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) + 8O2(g) P4O10(s) + 6H2O(g) If 15.0 g of phosphine reacts with sufficient oxygen, how many grams of P4O10 will be formed?
(Multiple Choice)
4.8/5  (44)
(44)
Nitrogen monoxide reacts with oxygen according to the following reaction: 2NO(g) + O2(g) 2NO2(g) If 12 moles of nitrogen monoxide are combined with 10 moles of oxygen, how many moles of NO2 should form?
(Multiple Choice)
4.9/5  (32)
(32)
The following reaction absorbs 393 kJ of heat for each mole of CO2 that reacts. CO2(g) C(s) + O2(g) This reaction
(Multiple Choice)
4.9/5  (41)
(41)
When mercury(II) oxide, a red crystalline solid, is heated, it decomposes to form liquid mercury and oxygen gas, according to the following equation: ___HgO(s) ___Hg(l) + ___O2(g) (unbalanced) Balance the equation and determine the mass of mercury that should be formed when 15.6 g of HgO is heated.
(Multiple Choice)
4.8/5  (34)
(34)
In the process of obtaining lead from PbS, or galena, the galena is "roasted" (heated in the presence of oxygen), so that the following reaction occurs: 2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g) If 35.2 g of PbS is mixed with 15.5 g of oxygen, how many grams of PbO should form?
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 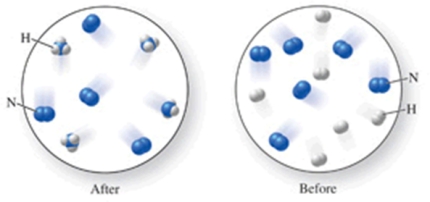
(Multiple Choice)
4.9/5  (35)
(35)
Consider the reaction between acetylene, C2H2, and oxygen in a welding torch: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) If 5.4 moles of acetylene react with sufficient oxygen, how many grams of CO2 should form?
(Multiple Choice)
4.7/5  (36)
(36)
A 2.50 g sample of pitted prunes was burned in a bomb calorimeter containing 2110 g of water.The temperature of the water increased from 22.50 C to 25.76 C.How much heat, in joules, did the prune sample release when it burned? [Cwater = 4.184 J/(g C)]
(Multiple Choice)
5.0/5  (38)
(38)
Nitrogen monoxide reacts with oxygen according to the following reaction: 2NO(g) + O2(g) 2NO2(g) If 10 moles of nitrogen monoxide are combined with 4 moles of oxygen, how many moles of NO2 should form?
(Multiple Choice)
4.9/5  (36)
(36)
The figure shows a molecular-level diagram of reactant molecules for the reaction: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) 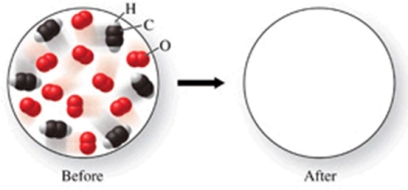 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
(Multiple Choice)
4.9/5  (33)
(33)
Aluminum reacts with oxygen according to the following reaction: 4Al(s) + 3O2(g) 2Al2O3(s) If 24 moles of aluminum are combined with 12 moles of oxygen, how many moles of Al2O3 should form?
(Multiple Choice)
5.0/5  (40)
(40)
What is the heat change when a 26.8 g sample of water [Cwater = 4.184 J/(g C)] is cooled from 75.6 C to 22.1 C?
(Multiple Choice)
4.9/5  (33)
(33)
Consider the following specific heats of metals. 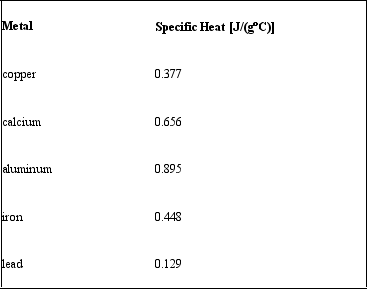 If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
(Multiple Choice)
4.8/5  (48)
(48)
Given the balanced equation 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 32.5 g of NH3 react with sufficient oxygen, how many grams of H2O should form?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 41 - 60 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)