Exam 7: Substitution Reactions: the Sn2 and Sn1 Reactions
Exam 1: Atoms and Molecules; Orbitals and Bonding64 Questions
Exam 2: Alkanes65 Questions
Exam 3: Alkenes and Alkynes70 Questions
Exam 4: Stereochemistry68 Questions
Exam 5: Rings60 Questions
Exam 6: Substituted Alkanes: Alkyl Halides, Alcohols, Amines, Ethers,thiols, and Thioethers68 Questions
Exam 7: Substitution Reactions: the Sn2 and Sn1 Reactions55 Questions
Exam 8: Elimination Reactions: the E1 and E2 Reactions45 Questions
Exam 9: Analytical Chemistry: Spectroscopy65 Questions
Exam 10: Electrophilic Additions to Alkenes68 Questions
Exam 11: More Additions to Bonds65 Questions
Exam 12: Radical Reactions65 Questions
Exam 13: Dienes and the Allyl System: 2p Orbitals in Conjugation68 Questions
Exam 14: Aromaticity66 Questions
Exam 15: Substitution Reactions of Aromatic Compounds68 Questions
Exam 16: Carbonyl Chemistry 1: Addition Reactions73 Questions
Exam 17: Carboxylic Acids66 Questions
Exam 18: Derivatives of Carboxylic Acids: Acyl Compounds68 Questions
Exam 19: Carbonyl Chemistry 2: Reactions at the Α Position71 Questions
Exam 20: Special Topic: Carbohydrates40 Questions
Exam 21: Special Topic: Bio-Organic Chemistry40 Questions
Exam 22: Special Topic: Amino Acids and Polyamino Acids Peptides and Proteins39 Questions
Exam 23: Special Topic: Reactions Controlled by Orbital Symmetry46 Questions
Exam 24: Special Topic: Intramolecular Reactions and Neighboring Group Participation40 Questions
Select questions type
The final step in the SN1 reaction of water with tert-butyl bromide to form tert-butyl alcohol is the deprotonation of the oxonium ion to give the alcohol plus hydronium:
 The relative energies of the starting materials and products in this step are expected to be very similar. Explain why in practice this reaction proceeds essentially to completion.
The relative energies of the starting materials and products in this step are expected to be very similar. Explain why in practice this reaction proceeds essentially to completion.
(Essay)
4.7/5  (41)
(41)
Which of the following statements about Lewis acids and bases is false?
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following compounds is the product of the reaction shown that displays retention of configuration compared to the starting material? 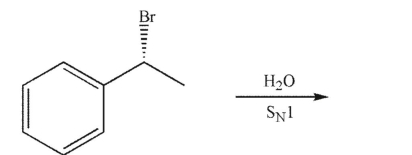
(Multiple Choice)
4.9/5  (35)
(35)
What sequence of reactions could be used for the following synthesis?
(Multiple Choice)
4.8/5  (38)
(38)
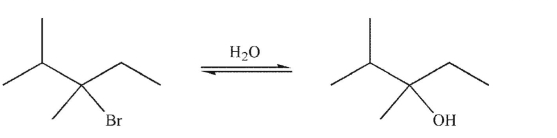
What is the most likely stereochemical outcome of the SN1 reaction shown here?
(Multiple Choice)
4.8/5  (45)
(45)
For the reaction profile shown below, which statement(s) could be made? 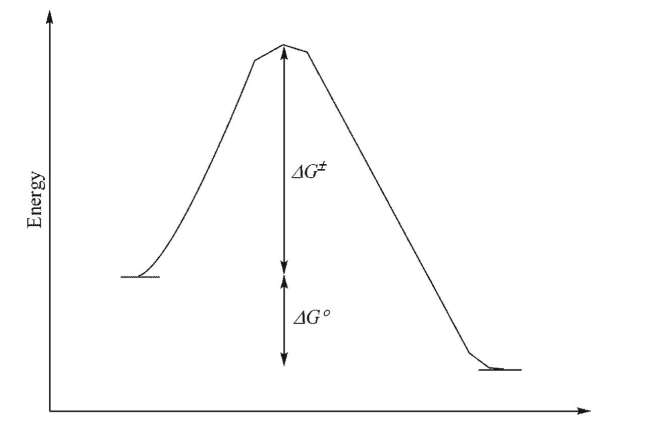 I.The transition state will look more like the starting materials than the products.
II.The reaction is endothermic.
III.The equilibrium constant for the forward reaction will be larger than 1.
IV.The activation barriers for the forward and reverse reactions are equal.
I.The transition state will look more like the starting materials than the products.
II.The reaction is endothermic.
III.The equilibrium constant for the forward reaction will be larger than 1.
IV.The activation barriers for the forward and reverse reactions are equal.
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following ideas best explains why SN2 reactions proceed with inversion of configuration?
(Multiple Choice)
4.7/5  (41)
(41)
Outline a multistep synthesis of the target molecule from the starting material shown.You may
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step. 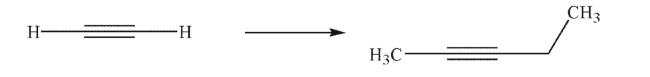
(Essay)
4.8/5  (44)
(44)
Identify the Lewis acid and the Lewis base in the reaction shown here.
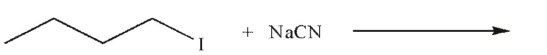
(Essay)
4.7/5  (43)
(43)
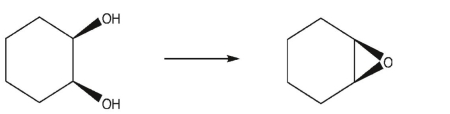
An intramolecular Williamson ether synthesis may be used to produce epoxides.Predict the
product of the following reaction conditions and provide a mechanism for the transformation. 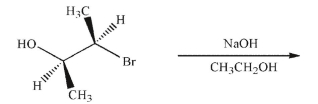
(Essay)
4.9/5  (38)
(38)
Chemicals such as dimethyl sulfate and iodomethane are considered biologically hazardous because:
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following electrophiles would undergo an SN2 reaction with thiolate ion (HS-) at the fastest rate?
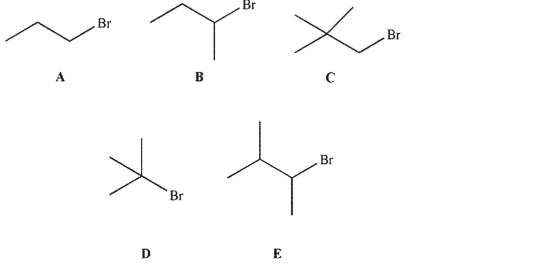
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following factors would not be expected to affect the entropy of a reaction?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 55 of 55
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)