Exam 7: Electron Structure of the Atom
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
Which of the following statements regarding spectra is incorrect?
(Multiple Choice)
4.7/5  (41)
(41)
Upon electrification, hydrogen produces a line spectrum with the following lines in the visible region of the electromagnetic spectrum. If the light emitted corresponds to transitions from the third (n = 3), fourth (n = 4), fifth (n = 5), or sixth (n = 6)energy level down to the second (n = 2), which transition corresponds to the emission of light with highest energy? 
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements regarding orbitals is correct?
(Multiple Choice)
4.8/5  (35)
(35)
Some elements have electron configurations that deviate from normal electron filling rules. Which element has the ground-state electron configuration [Ar]4s13d10?
(Multiple Choice)
4.7/5  (35)
(35)
When the elements lithium and fluorine react, they become ions and form the ionic compound LiF: 2Li(s)+ F2(g)→ 2LiF(s)Which of the following atoms decreases in radius as reactants turn to products?
(Multiple Choice)
4.9/5  (50)
(50)
Write the element symbol for the element with the abbreviated electron configuration [Ne]3s23p2.
(Short Answer)
4.7/5  (36)
(36)
The following orbital diagram corresponds to the element ________. 
(Multiple Choice)
4.9/5  (38)
(38)
Write the element symbol for the element with the abbreviated electron configuration [Ar]4s23d8.
(Short Answer)
4.8/5  (32)
(32)
The following orbital diagram corresponds to the element ________. 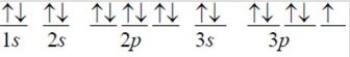
(Multiple Choice)
5.0/5  (33)
(33)
Your friend wrote the electron configuration of selenium as 1s22s22p63s23p64s24d104p2. Describe how you would correct your friend's electron configuration.
(Short Answer)
4.7/5  (40)
(40)
Explain in terms of atomic structure why more energy is required to remove one electron from a fluorine atom than from a chlorine atom.
(Essay)
4.8/5  (32)
(32)
What is the ground-state electron configuration for the calcium ion, Ca2+?
(Multiple Choice)
4.8/5  (38)
(38)
Rank the following types of electromagnetic radiation from lowest frequency to highest frequency: visible, ultraviolet, microwave, infrared, x-ray.
(Multiple Choice)
4.8/5  (41)
(41)
When completing the orbital diagram for the element phosphorus, which of the following statements is correct?
(Multiple Choice)
4.9/5  (52)
(52)
Write the element symbol for the element with the abbreviated electron configuration [Ne]3s23p4.
(Short Answer)
4.9/5  (45)
(45)
The color of visible light with the longest wavelength is violet.
(True/False)
4.9/5  (34)
(34)
Write the element symbol for the element with the abbreviated electron configuration [Ar]4s23d6.
(Short Answer)
4.9/5  (41)
(41)
Showing 41 - 60 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)