Exam 7: Electron Structure of the Atom
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
Elements that have six electrons in the highest-energy p sublevel in their ground state are called ________.
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following statements regarding electromagnetic radiation is incorrect?
(Multiple Choice)
4.9/5  (40)
(40)
Which element has two completely filled p sublevels, and four electrons in its other p sublevel in its ground-state electron configuration?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements regarding orbitals is correct?
(Multiple Choice)
4.7/5  (32)
(32)
Upon electrification, hydrogen produces a characteristic line spectrum consisting of four lines in the visible region of the electromagnetic spectrum. The light emitted in different regions of the visible spectrum corresponds to transitions from the third (n = 3), fourth (n = 4), fifth (n = 5), or sixth (n = 6)energy level down to the second (n = 2). Which transition corresponds to the violet line in the hydrogen spectrum?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is the correct ground state electron configuration for a silicon atom?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following is the correct orbital diagram for phosphorus?
(Multiple Choice)
4.8/5  (38)
(38)
Upon electrification, hydrogen produces a line spectrum with the following lines in the visible region of the electromagnetic spectrum. If the light emitted corresponds to transitions from the third (n = 3), fourth (n = 4), fifth (n = 5), or sixth (n = 6)energy level down to the second (n = 2), which transition corresponds to the emission of light with lowest frequency? 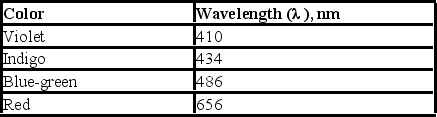
(Multiple Choice)
4.8/5  (46)
(46)
Some elements have electron configurations that deviate from normal electron filling rules. Which element has the ground-state electron configuration [Kr]4d10?
(Multiple Choice)
4.8/5  (34)
(34)
Rank the following elements in order of increasing atomic size: Al, Ba, O, C
(Multiple Choice)
5.0/5  (40)
(40)
What is the abbreviated ground-state electron configuration for Sc?
(Multiple Choice)
4.9/5  (39)
(39)
What is the abbreviated ground-state electron configuration for Co?
(Multiple Choice)
4.8/5  (45)
(45)
In the Bohr model of the hydrogen atom, when an electron absorbs energy, it moves into a higher energy orbit.
(True/False)
4.8/5  (41)
(41)
The wavelength of the blue light given off by a mercury vapor street lamp is 436 nm. What is the frequency of this light in hertz (s-1)?
(Multiple Choice)
4.9/5  (32)
(32)
The maximum number of electrons in the 2nd principal energy level is 8.
(True/False)
4.9/5  (33)
(33)
Which of the following statements regarding the Bohr model of the hydrogen atom is incorrect?
(Multiple Choice)
4.9/5  (36)
(36)
The formula of the ionic compound that would be formed when magnesium and fluorine react is:
(Multiple Choice)
4.8/5  (34)
(34)
List the following colors of visible light from lowest energy to highest energy: green, blue, yellow, red, violet.
(Multiple Choice)
4.9/5  (35)
(35)
Showing 81 - 100 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)