Exam 22: Coordination Chemistry
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Octahedral complexes can exhibit geometric and optical isomerism.
(True/False)
4.7/5  (33)
(33)
How many geometric isomers can the following square-planar complex have? 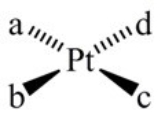
(Multiple Choice)
4.9/5  (33)
(33)
In K4[Fe(CN)6], how many 3d electrons does the iron atom have?
(Multiple Choice)
4.7/5  (39)
(39)
If the plane of polarization is rotated to the right then the isomer is ________.
(Short Answer)
4.8/5  (36)
(36)
What is the name of the atom in a ligand that is bound directly to the metal atom?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following ligands has two different types of donor atoms?
(Multiple Choice)
4.9/5  (33)
(33)
Iron(III) forms an octahedral complex with the ligand CN-. How many unpaired electrons are in the d orbitals of iron?
(Multiple Choice)
4.8/5  (40)
(40)
The following energy-level diagram could correspond to which coordination compound? 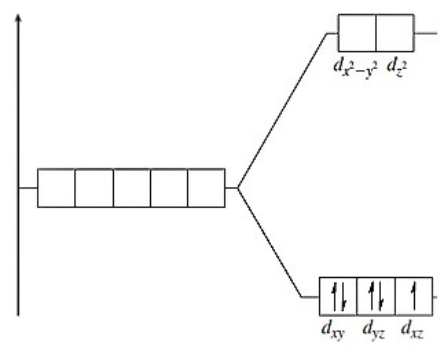
(Multiple Choice)
4.7/5  (42)
(42)
Write the formula for diamminedichloroethylenediaminecobalt(III) bromide.
(Multiple Choice)
4.7/5  (40)
(40)
In the coordination compound [Pt(NH3)2Cl2], the coordination number and oxidation number of the central atom are ________ and ________, respectively.
(Multiple Choice)
4.7/5  (32)
(32)
Which of these diagrams represent the crystal field splitting between d orbitals in a tetrahedral complex?
(Multiple Choice)
4.9/5  (31)
(31)
An equimolar mixture (50:50 ratio) of two enantiomers is called a(n) ________ ________.
(Short Answer)
4.8/5  (43)
(43)
The maximum oxidation state of an element in the first transition series never exceeds its group number.
(True/False)
4.8/5  (30)
(30)
The ion [Co(NH3)6]2+ is octahedral and high spin. This complex is
(Multiple Choice)
4.8/5  (39)
(39)
In the coordination compound [Co(en)2Cl2]Cl, the coordination number and oxidation number of the central atom are ________ and ________, respectively.
(Multiple Choice)
4.8/5  (43)
(43)
Showing 101 - 120 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)