Exam 22: Coordination Chemistry
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which of the following ions could exist in only the high-spin state in an octahedral complex?
(Multiple Choice)
4.9/5  (37)
(37)
The systematic name of the coordination compound K2[Co(H2O)2I4] is potassium diaquotetraiodocobaltate(II).
(True/False)
4.9/5  (39)
(39)
In complexes of transition metals, the maximum coordination number of the metal is equal to its number of d electrons.
(True/False)
4.8/5  (36)
(36)
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 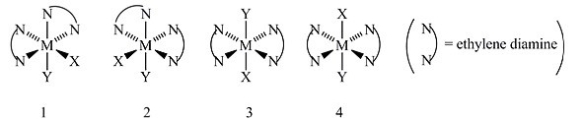 Which, if any, of the following pairs are optical isomers?
Which, if any, of the following pairs are optical isomers?
(Multiple Choice)
4.8/5  (46)
(46)
The following energy-level diagram could correspond to which coordination compound? 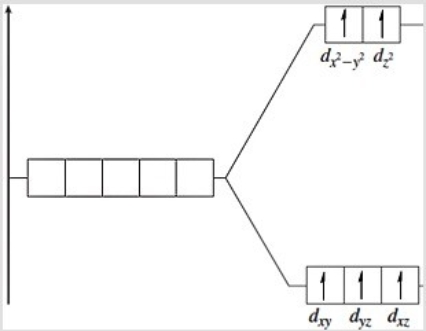
(Multiple Choice)
4.9/5  (36)
(36)
The net rotation of plane-polarized light by a racemic mixture is ________.
(Multiple Choice)
4.9/5  (42)
(42)
The atom in a ligand that is bound directly to the metal atom is known as the ________ ________.
(Short Answer)
4.8/5  (30)
(30)
Which of these square planar complex ions can have cis-trans isomers?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following octahedral complexes should have the largest crystal field splitting energy, Δ?
(Multiple Choice)
4.9/5  (39)
(39)
What is the name that refers to the number of donor atoms surrounding the central metal atom in a complex ion?
(Multiple Choice)
4.8/5  (41)
(41)
In a reaction between a metal ion and a group of anions or a polar molecule, the metal ion acts as a ________ ________.
(Short Answer)
4.9/5  (37)
(37)
In the coordination compound [Cr(NH3)2(en)Cl2]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom, respectively, are
(Multiple Choice)
4.9/5  (35)
(35)
How many unpaired electrons are there in the complex ion [Mn(CN)6]3-?
(Multiple Choice)
4.7/5  (36)
(36)
What name is given to the species that has the ability to hold the metal atom, in the complex ion, like a claw?
(Multiple Choice)
4.9/5  (36)
(36)
In the complex ion [Co(en)2Br2]+, what is the oxidation number of Co?
(Multiple Choice)
4.9/5  (37)
(37)
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar). 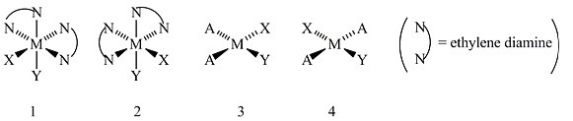 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 61 - 80 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)