Exam 22: Coordination Chemistry
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which name could correspond to the following coordination compound, where M represents a transition metal cation and L represents a ligand? 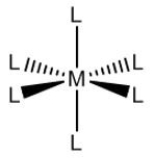
(Multiple Choice)
4.8/5  (38)
(38)
How many donor atoms enable EDTA to form a stable complex ion with lead?
(Multiple Choice)
4.8/5  (35)
(35)
The numbers of geometrical isomers and optical isomers of the complex ion [Co(en)3]3+ are, respectively,
(Multiple Choice)
4.7/5  (40)
(40)
What type of structure is exhibited by most of the transition metals?
(Multiple Choice)
4.8/5  (33)
(33)
Which of these electron energy level patterns would absorb light with the shortest wavelength?
(Multiple Choice)
4.9/5  (41)
(41)
In the coordination compound [Cr(NH3)(en)2Cl]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom are, respectively,
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following ions is least likely to form colored compounds?
(Multiple Choice)
5.0/5  (29)
(29)
What is the name that refers to the number of donor atoms surrounding the central metal atom in a complex ion?
(Short Answer)
4.9/5  (44)
(44)
A complex ion that undergoes a very slow exchange reaction is called an inert complex.
(True/False)
4.7/5  (41)
(41)
What is the electron configuration of a ground-state Cr atom?
(Multiple Choice)
4.9/5  (38)
(38)
Which response gives the correct coordination number (C.N.) and oxidation number (O.N.) of the transition metal atom in [Co(NH3)2(H2O)2Cl2]+?
(Multiple Choice)
4.8/5  (43)
(43)
________ is the name given to compounds that have more than one way to arrange the ligands around the central atom and have distinctly different physical and chemical properties.
(Short Answer)
4.7/5  (43)
(43)
What are the two types of stereoisomers that coordination compounds may exhibit?
(Short Answer)
4.8/5  (35)
(35)
Coordination compounds may exhibit ________ and ________ types of stereoisomerism.
(Short Answer)
4.7/5  (36)
(36)
One of the uses of EDTA is in the treatment of ________ ________.
(Short Answer)
4.9/5  (34)
(34)
Showing 41 - 60 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)